Miruna Cretu
SynCoGen: Synthesizable 3D Molecule Generation via Joint Reaction and Coordinate Modeling
Jul 16, 2025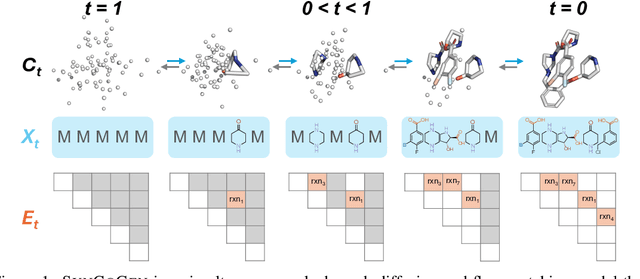
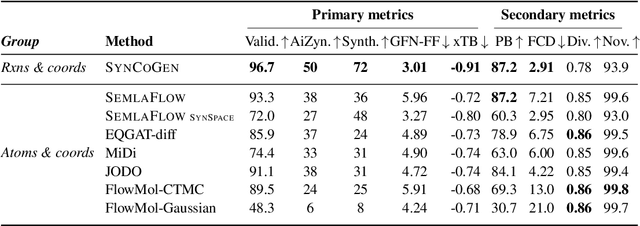
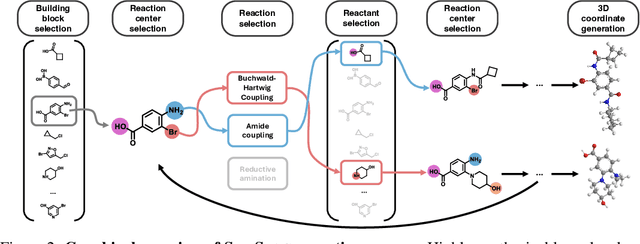
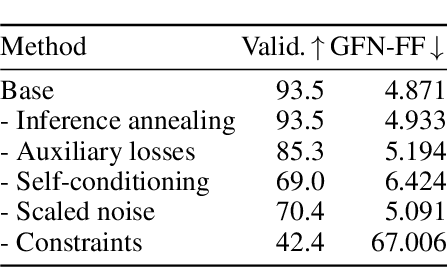
Abstract:Ensuring synthesizability in generative small molecule design remains a major challenge. While recent developments in synthesizable molecule generation have demonstrated promising results, these efforts have been largely confined to 2D molecular graph representations, limiting the ability to perform geometry-based conditional generation. In this work, we present SynCoGen (Synthesizable Co-Generation), a single framework that combines simultaneous masked graph diffusion and flow matching for synthesizable 3D molecule generation. SynCoGen samples from the joint distribution of molecular building blocks, chemical reactions, and atomic coordinates. To train the model, we curated SynSpace, a dataset containing over 600K synthesis-aware building block graphs and 3.3M conformers. SynCoGen achieves state-of-the-art performance in unconditional small molecule graph and conformer generation, and the model delivers competitive performance in zero-shot molecular linker design for protein ligand generation in drug discovery. Overall, this multimodal formulation represents a foundation for future applications enabled by non-autoregressive molecular generation, including analog expansion, lead optimization, and direct structure conditioning.
TABASCO: A Fast, Simplified Model for Molecular Generation with Improved Physical Quality
Jul 01, 2025Abstract:State-of-the-art models for 3D molecular generation are based on significant inductive biases, SE(3), permutation equivariance to respect symmetry and graph message-passing networks to capture local chemistry, yet the generated molecules still struggle with physical plausibility. We introduce TABASCO which relaxes these assumptions: The model has a standard non-equivariant transformer architecture, treats atoms in a molecule as sequences and reconstructs bonds deterministically after generation. The absence of equivariant layers and message passing allows us to significantly simplify the model architecture and scale data throughput. On the GEOM-Drugs benchmark TABASCO achieves state-of-the-art PoseBusters validity and delivers inference roughly 10x faster than the strongest baseline, while exhibiting emergent rotational equivariance despite symmetry not being hard-coded. Our work offers a blueprint for training minimalist, high-throughput generative models suited to specialised tasks such as structure- and pharmacophore-based drug design. We provide a link to our implementation at github.com/carlosinator/tabasco.
SynFlowNet: Towards Molecule Design with Guaranteed Synthesis Pathways
May 02, 2024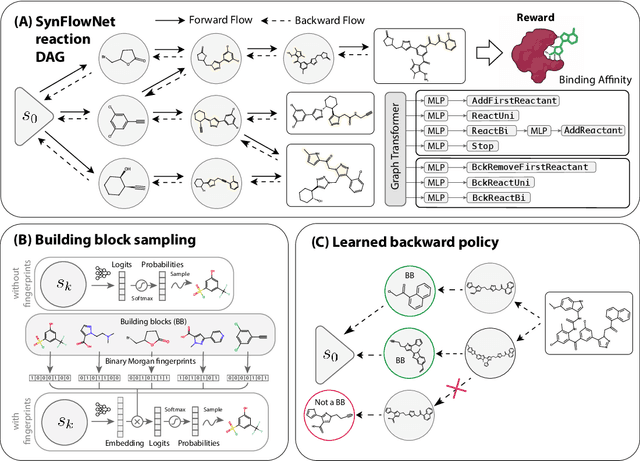

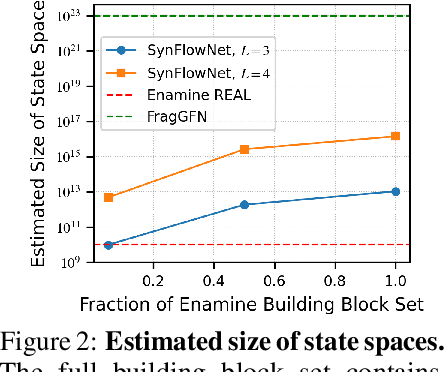
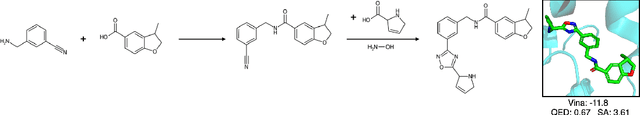
Abstract:Recent breakthroughs in generative modelling have led to a number of works proposing molecular generation models for drug discovery. While these models perform well at capturing drug-like motifs, they are known to often produce synthetically inaccessible molecules. This is because they are trained to compose atoms or fragments in a way that approximates the training distribution, but they are not explicitly aware of the synthesis constraints that come with making molecules in the lab. To address this issue, we introduce SynFlowNet, a GFlowNet model whose action space uses chemically validated reactions and reactants to sequentially build new molecules. We evaluate our approach using synthetic accessibility scores and an independent retrosynthesis tool. SynFlowNet consistently samples synthetically feasible molecules, while still being able to find diverse and high-utility candidates. Furthermore, we compare molecules designed with SynFlowNet to experimentally validated actives, and find that they show comparable properties of interest, such as molecular weight, SA score and predicted protein binding affinity.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge