Michael B. Mayhew
Optimization of genomic classifiers for clinical deployment: evaluation of Bayesian optimization for identification of predictive models of acute infection and in-hospital mortality
Mar 27, 2020


Abstract:Acute infection, if not rapidly and accurately detected, can lead to sepsis, organ failure and even death. Currently, detection of acute infection as well as assessment of a patient's severity of illness are based on imperfect (and often superficial) measures of patient physiology. Characterization of a patient's immune response by quantifying expression levels of key genes from blood represents a potentially more timely and precise means of accomplishing both tasks. Machine learning methods provide a platform for development of deployment-ready classification models robust to the smaller, more heterogeneous datasets typical of healthcare. Identification of promising classifiers is dependent, in part, on hyperparameter optimization (HO), for which a number of approaches including grid search, random sampling and Bayesian optimization have been shown to be effective. In this analysis, we compare HO approaches for the development of diagnostic classifiers of acute infection and in-hospital mortality from gene expression of 29 diagnostic markers. Our comprehensive analysis of a multi-study patient cohort evaluates HO for three different classifier types and over a range of different optimization settings. Consistent with previous research, we find that Bayesian optimization is more efficient than grid search or random sampling-based methods, identifying promising classifiers with fewer evaluated hyperparameter configurations. However, we also find evidence of a lack of correspondence between internal and external validation performance of selected classifiers that complicates model selection for deployment as well as stymies development of clear-cut, practical guidelines for HO application in healthcare. We highlight the need for additional considerations about patient heterogeneity, dataset partitioning and optimization setup when applying HO methods in the healthcare context.
Modeling sepsis progression using hidden Markov models
Jan 09, 2018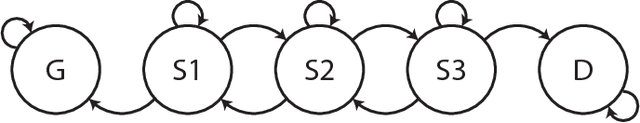
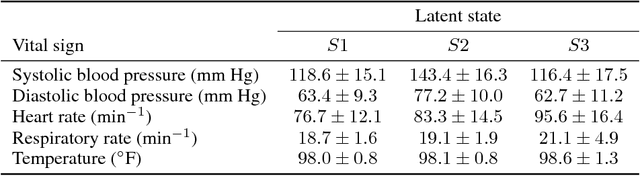
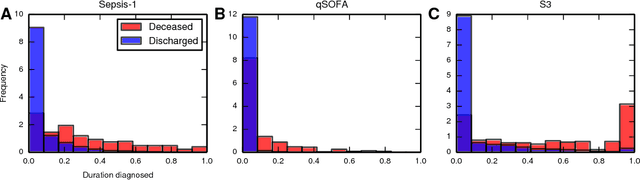
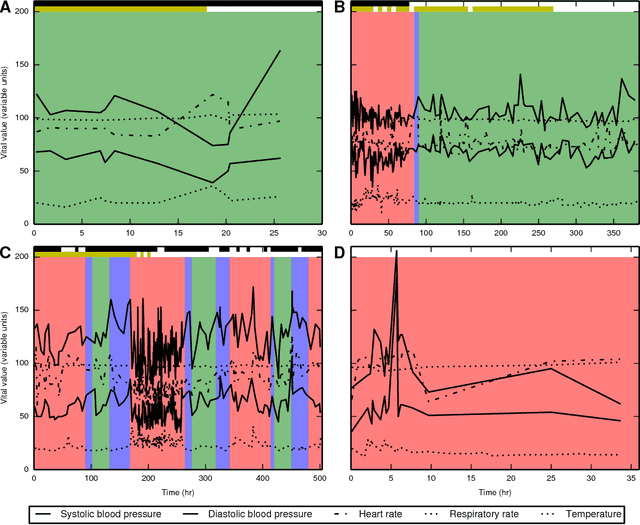
Abstract:Characterizing a patient's progression through stages of sepsis is critical for enabling risk stratification and adaptive, personalized treatment. However, commonly used sepsis diagnostic criteria fail to account for significant underlying heterogeneity, both between patients as well as over time in a single patient. We introduce a hidden Markov model of sepsis progression that explicitly accounts for patient heterogeneity. Benchmarked against two sepsis diagnostic criteria, the model provides a useful tool to uncover a patient's latent sepsis trajectory and to identify high-risk patients in whom more aggressive therapy may be indicated.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge