Mehrad Jaloli
Model Predictive Control (MPC) of an Artificial Pancreas with Data-Driven Learning of Multi-Step-Ahead Blood Glucose Predictors
Jul 22, 2023Abstract:We present the design and \textit{in-silico} evaluation of a closed-loop insulin delivery algorithm to treat type 1 diabetes (T1D) consisting in a data-driven multi-step-ahead blood glucose (BG) predictor integrated into a Linear Time-Varying (LTV) Model Predictive Control (MPC) framework. Instead of identifying an open-loop model of the glucoregulatory system from available data, we propose to directly fit the entire BG prediction over a predefined prediction horizon to be used in the MPC, as a nonlinear function of past input-ouput data and an affine function of future insulin control inputs. For the nonlinear part, a Long Short-Term Memory (LSTM) network is proposed, while for the affine component a linear regression model is chosen. To assess benefits and drawbacks when compared to a traditional linear MPC based on an auto-regressive with exogenous (ARX) input model identified from data, we evaluated the proposed LSTM-MPC controller in three simulation scenarios: a nominal case with 3 meals per day, a random meal disturbances case where meals were generated with a recently published meal generator, and a case with 25$\%$ decrease in the insulin sensitivity. Further, in all the scenarios, no feedforward meal bolus was administered. For the more challenging random meal generation scenario, the mean $\pm$ standard deviation percent time in the range 70-180 [mg/dL] was 74.99 $\pm$ 7.09 vs. 54.15 $\pm$ 14.89, the mean $\pm$ standard deviation percent time in the tighter range 70-140 [mg/dL] was 47.78$\pm$8.55 vs. 34.62 $\pm$9.04, while the mean $\pm$ standard deviation percent time in sever hypoglycemia, i.e., $<$ 54 [mg/dl] was 1.00$\pm$3.18 vs. 9.45$\pm$11.71, for our proposed LSTM-MPC controller and the traditional ARX-MPC, respectively. Our approach provided accurate predictions of future glucose concentrations and good closed-loop performances of the overall MPC controller.
Modeling Physical Activity Impact on Glucose Dynamics in People with Type 1 Diabetes for a Fully Automated Artificial Pancreas
Jul 16, 2023



Abstract:In this paper, models of the blood glucose (BG) dynamics in people with Type 1 diabetes (T1D) in response to moderate intensity aerobic activity are derived from physiology-based first principles and system identification experiments. We show that by enhancing insulin-dependent glucose utilization by the tissues in two phases, a rapid short-term increase in insulin-independent glucose clearance and augmented glucose uptake, and a long-term sustained increase sensitivity to insulin action, a metabolic model able to reproduce the effects of activity on glucose disposal is obtained. Second, a control-oriented transfer function model is proposed to predict the BG response to an exercise bout modeled as a step change in heart rate (HR). Results comparing model predictions with actual patients data collected in a series of experimental sessions including physical activity (PA) are presented. The findings will contribute to the design of a fully automated closed-loop for improved glucose control in conditions of daily life for people with T1D.
Neurological Status Classification Using Convolutional Neural Network
Apr 01, 2021
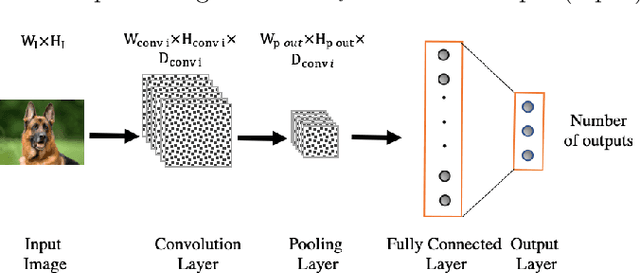
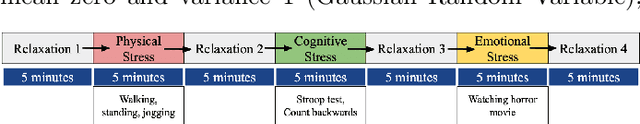
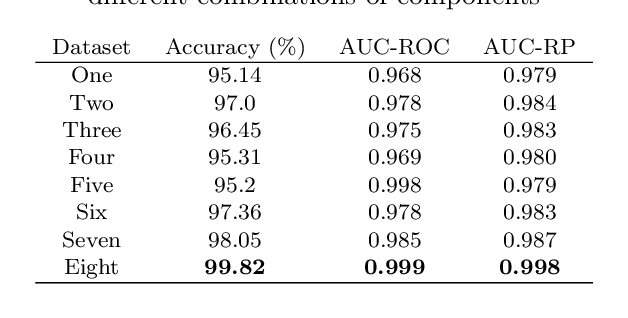
Abstract:In this study we show that a Convolutional Neural Network (CNN) model is able to accuratelydiscriminate between 4 different phases of neurological status in a non-Electroencephalogram(EEG) dataset recorded in an experiment in which subjects are exposed to physical, cognitiveand emotional stress. We demonstrate that the proposed model is able to obtain 99.99% AreaUnder the Curve (AUC) of Receiver Operation characteristic (ROC) and 99.82% classificationaccuracy on the test dataset. Furthermore, for comparison, we show that our models outperformstraditional classification methods such as SVM, and RF. Finally, we show the advantage of CNN models, in comparison to other methods, in robustness to noise by 97.46% accuracy on a noisy dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge