Mahmood A. Rashid
Guided macro-mutation in a graded energy based genetic algorithm for protein structure prediction
Mar 07, 2016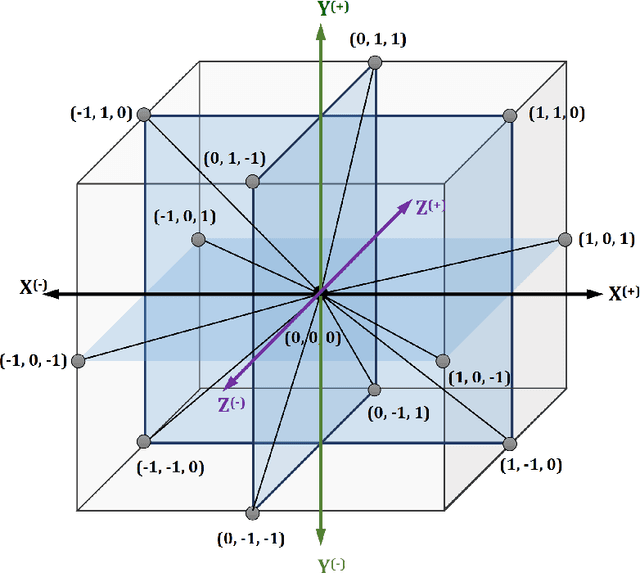
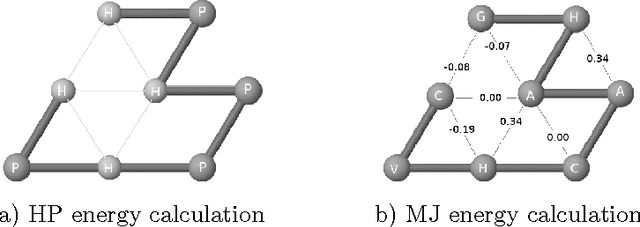
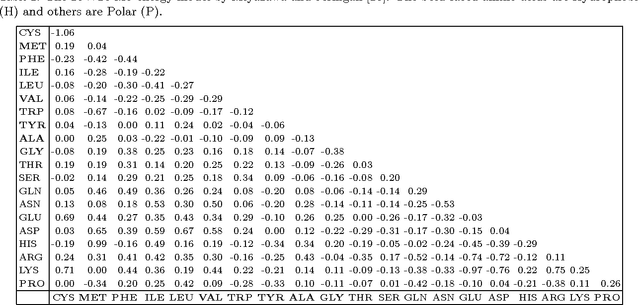
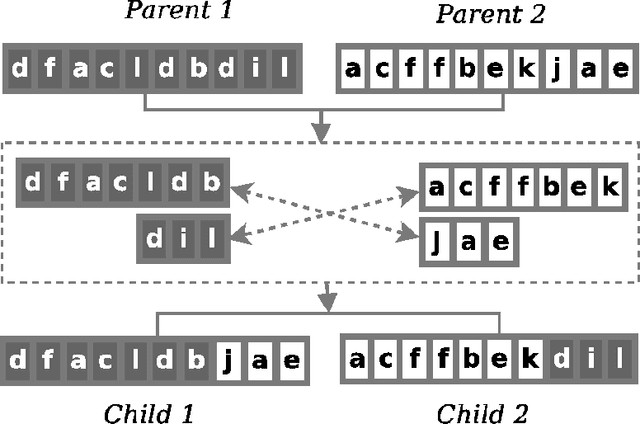
Abstract:Protein structure prediction is considered as one of the most challenging and computationally intractable combinatorial problem. Thus, the efficient modeling of convoluted search space, the clever use of energy functions, and more importantly, the use of effective sampling algorithms become crucial to address this problem. For protein structure modeling, an off-lattice model provides limited scopes to exercise and evaluate the algorithmic developments due to its astronomically large set of data-points. In contrast, an on-lattice model widens the scopes and permits studying the relatively larger proteins because of its finite set of data-points. In this work, we took the full advantage of an on-lattice model by using a face-centered-cube lattice that has the highest packing density with the maximum degree of freedom. We proposed a graded energy-strategically mixes the Miyazawa-Jernigan (MJ) energy with the hydrophobic-polar (HP) energy-based genetic algorithm (GA) for conformational search. In our application, we introduced a 2x2 HP energy guided macro-mutation operator within the GA to explore the best possible local changes exhaustively. Conversely, the 20x20 MJ energy model-the ultimate objective function of our GA that needs to be minimized-considers the impacts amongst the 20 different amino acids and allow searching the globally acceptable conformations. On a set of benchmark proteins, our proposed approach outperformed state-of-the-art approaches in terms of the free energy levels and the root-mean-square deviations.
* 29 pages. arXiv admin note: text overlap with arXiv:1311.3840
Mixing Energy Models in Genetic Algorithms for On-Lattice Protein Structure Prediction
Nov 15, 2013



Abstract:Protein structure prediction (PSP) is computationally a very challenging problem. The challenge largely comes from the fact that the energy function that needs to be minimised in order to obtain the native structure of a given protein is not clearly known. A high resolution 20x20 energy model could better capture the behaviour of the actual energy function than a low resolution energy model such as hydrophobic polar. However, the fine grained details of the high resolution interaction energy matrix are often not very informative for guiding the search. In contrast, a low resolution energy model could effectively bias the search towards certain promising directions. In this paper, we develop a genetic algorithm that mainly uses a high resolution energy model for protein structure evaluation but uses a low resolution HP energy model in focussing the search towards exploring structures that have hydrophobic cores. We experimentally show that this mixing of energy models leads to significant lower energy structures compared to the state-of-the-art results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge