Linxia Gu
A Comprehensive Review of Generative AI in Healthcare
Oct 01, 2023



Abstract:The advancement of Artificial Intelligence (AI) has catalyzed revolutionary changes across various sectors, notably in healthcare. Among the significant developments in this field are the applications of generative AI models, specifically transformers and diffusion models. These models have played a crucial role in analyzing diverse forms of data, including medical imaging (encompassing image reconstruction, image-to-image translation, image generation, and image classification), protein structure prediction, clinical documentation, diagnostic assistance, radiology interpretation, clinical decision support, medical coding, and billing, as well as drug design and molecular representation. Such applications have enhanced clinical diagnosis, data reconstruction, and drug synthesis. This review paper aims to offer a thorough overview of the generative AI applications in healthcare, focusing on transformers and diffusion models. Additionally, we propose potential directions for future research to tackle the existing limitations and meet the evolving demands of the healthcare sector. Intended to serve as a comprehensive guide for researchers and practitioners interested in the healthcare applications of generative AI, this review provides valuable insights into the current state of the art, challenges faced, and prospective future directions.
Deep Learning-based Prediction of Stress and Strain Maps in Arterial Walls for Improved Cardiovascular Risk Assessment
Aug 03, 2023



Abstract:This study investigated the potential of end-to-end deep learning tools as a more effective substitute for FEM in predicting stress-strain fields within 2D cross sections of arterial wall. We first proposed a U-Net based fully convolutional neural network (CNN) to predict the von Mises stress and strain distribution based on the spatial arrangement of calcification within arterial wall cross-sections. Further, we developed a conditional generative adversarial network (cGAN) to enhance, particularly from the perceptual perspective, the prediction accuracy of stress and strain field maps for arterial walls with various calcification quantities and spatial configurations. On top of U-Net and cGAN, we also proposed their ensemble approaches, respectively, to further improve the prediction accuracy of field maps. Our dataset, consisting of input and output images, was generated by implementing boundary conditions and extracting stress-strain field maps. The trained U-Net models can accurately predict von Mises stress and strain fields, with structural similarity index scores (SSIM) of 0.854 and 0.830 and mean squared errors of 0.017 and 0.018 for stress and strain, respectively, on a reserved test set. Meanwhile, the cGAN models in a combination of ensemble and transfer learning techniques demonstrate high accuracy in predicting von Mises stress and strain fields, as evidenced by SSIM scores of 0.890 for stress and 0.803 for strain. Additionally, mean squared errors of 0.008 for stress and 0.017 for strain further support the model's performance on a designated test set. Overall, this study developed a surrogate model for finite element analysis, which can accurately and efficiently predict stress-strain fields of arterial walls regardless of complex geometries and boundary conditions.
Prediction of stent under-expansion in calcified coronary arteries using machine-learning on intravascular optical coherence tomography
May 16, 2022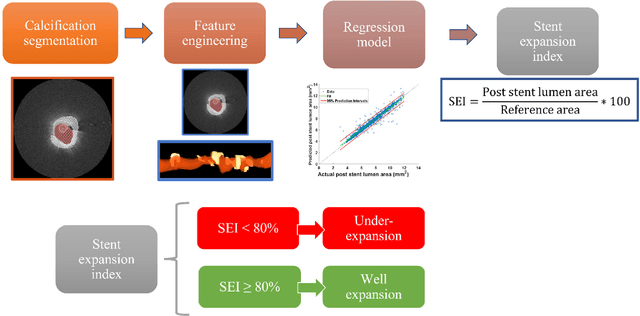
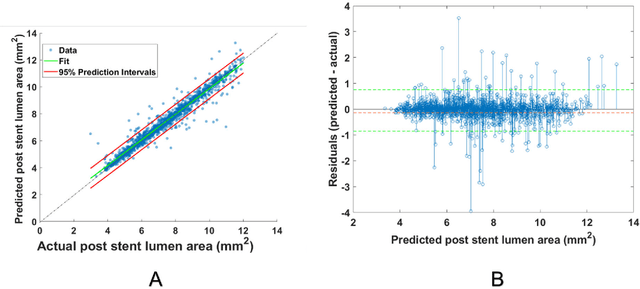
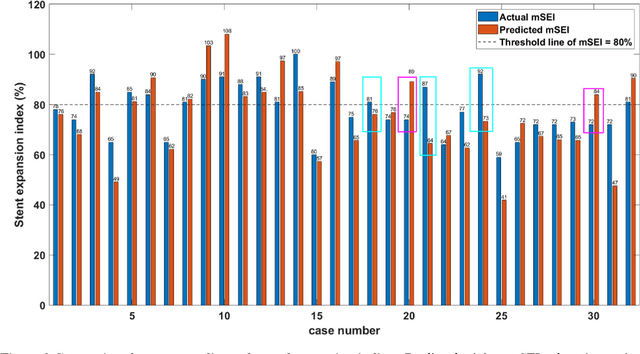
Abstract:BACKGROUND Careful evaluation of the risk of stent under-expansions before the intervention will aid treatment planning, including the application of a pre-stent plaque modification strategy. OBJECTIVES It remains challenging to achieve a proper stent expansion in the presence of severely calcified coronary lesions. Building on our work in deep learning segmentation, we created an automated machine learning approach that uses lesion attributes to predict stent under-expansion from pre-stent images, suggesting the need for plaque modification. METHODS Pre- and post-stent intravascular optical coherence tomography image data were obtained from 110 coronary lesions. Lumen and calcifications in pre-stent images were segmented using deep learning, and numerous features per lesion were extracted. We analyzed stent expansion along the lesion, enabling frame, segmental, and whole-lesion analyses. We trained regression models to predict the poststent lumen area and then to compute the stent expansion index (SEI). Stents with an SEI < or >/= 80% were classified as "under-expanded" and "well-expanded," respectively. RESULTS Best performance (root-mean-square-error = 0.04+/-0.02 mm2, r = 0.94+/-0.04, p < 0.0001) was achieved when we used features from both the lumen and calcification to train a Gaussian regression model for a segmental analysis over a segment length of 31 frames. Under-expansion classification results (AUC=0.85+/-0.02) were significantly improved over other approaches. CONCLUSIONS We used calcifications and lumen features to identify lesions at risk of stent under-expansion. Results suggest that the use of pre-stent images can inform physicians of the need to apply plaque modification approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge