Katie Walsh
Electroanatomic Mapping to determine Scar Regions in patients with Atrial Fibrillation
Oct 23, 2022Abstract:Left atrial voltage maps are routinely acquired during electroanatomic mapping in patients undergoing catheter ablation for atrial fibrillation. For patients, who have prior catheter ablation when they are in sinus rhythm, the voltage map can be used to identify low voltage areas using a threshold of 0.2 - 0.45 mV. However, such a voltage threshold for maps acquired during atrial fibrillation has not been well established. A prerequisite for defining a voltage threshold is to maximize the topologically matched low voltage areas between the electroanatomic mapping acquired during atrial fibrillation and sinus rhythm. This paper demonstrates a new technique to improve the sensitivity and specificity of the matched low voltage areas. This is achieved by computing omni-directional bipolar voltages and applying Gaussian Process Regression based interpolation to derive the AF map. The proposed method is evaluated on a test cohort of 7 male patients, and a total of 46,589 data points were included in analysis. The low voltage areas in the posterior left atrium and pulmonary vein junction are determined using the standard method and the proposed method. Overall, the proposed method showed patient-specific sensitivity and specificity in matching low voltage areas of 75.70% and 65.55% for a geometric mean of 70.69%. On average, there was an improvement of 3.00% in the geometric mean, 7.88% improvement in sensitivity, 0.30% improvement in specificity compared to the standard method. The results show that the proposed method is an improvement in matching low voltage areas. This may help develop the voltage threshold to better identify low voltage areas in the left atrium for patients in atrial fibrillation.
Patient-Specific Heart Model Towards Atrial Fibrillation
Oct 23, 2022
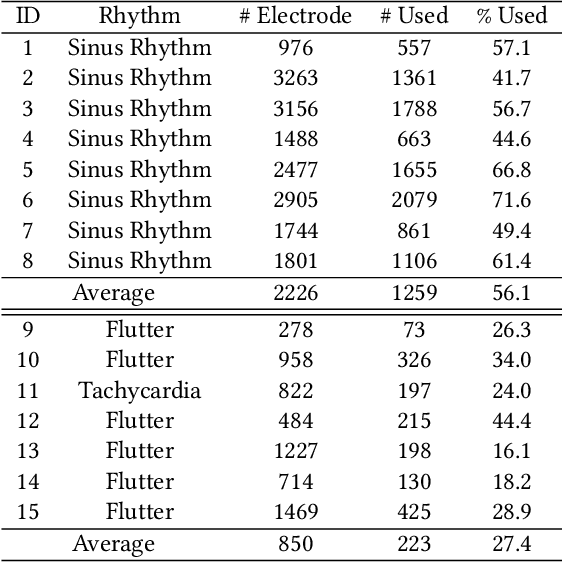
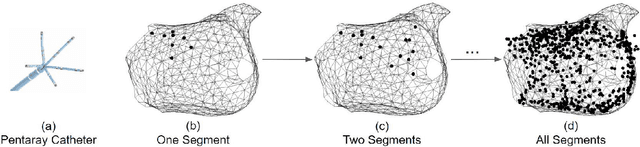
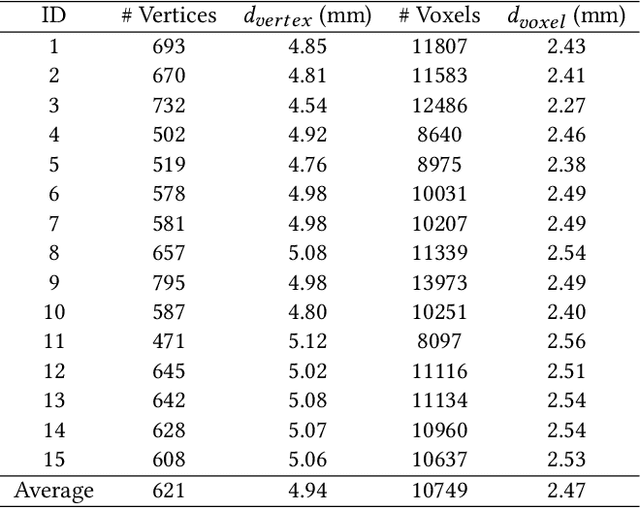
Abstract:Atrial fibrillation is a heart rhythm disorder that affects tens of millions people worldwide. The most effective treatment is catheter ablation. This involves irreversible heating of abnormal cardiac tissue facilitated by electroanatomical mapping. However, it is difficult to consistently identify the triggers and sources that may initiate or perpetuate atrial fibrillation due to its chaotic behavior. We developed a patient-specific computational heart model that can accurately reproduce the activation patterns to help in localizing these triggers and sources. Our model has high spatial resolution, with whole-atrium temporal synchronous activity, and has patient-specific accurate electrophysiological activation patterns. A total of 15 patients data were processed: 8 in sinus rhythm, 6 in atrial flutter and 1 in atrial tachycardia. For resolution, the average simulation geometry voxel is a cube of 2.47 mm length. For synchrony, the model takes in about 1,500 local electrogram recordings, optimally fits parameters to the individual's atrium geometry and then generates whole-atrium activation patterns. For accuracy, the average local activation time error is 5.47 ms for sinus rhythm, 10.97 ms for flutter and tachycardia; and the average correlation is 0.95 for sinus rhythm, 0.81 for flutter and tachycardia. This promising result demonstrates our model is an effective building block in capturing more complex rhythms such as atrial fibrillation to guide physicians for effective ablation therapy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge