Johannes Sarnthein
Klinik für Neurochirurgie, UniversitätsSpital und Universität Zürich
A Spiking Neural Network for detecting High Frequency Oscillations in the intraoperative ECoG
Nov 17, 2020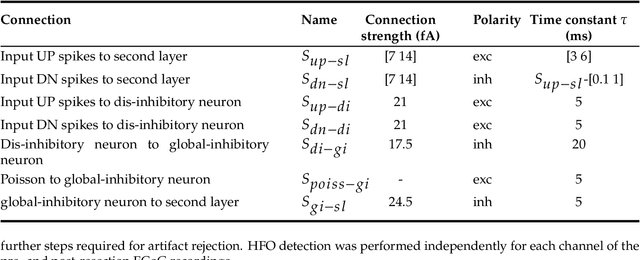
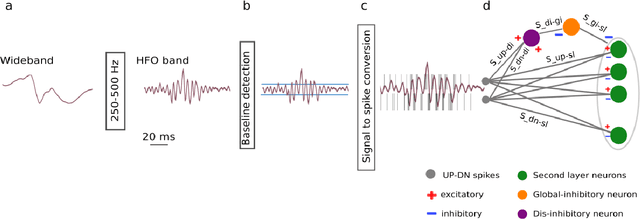

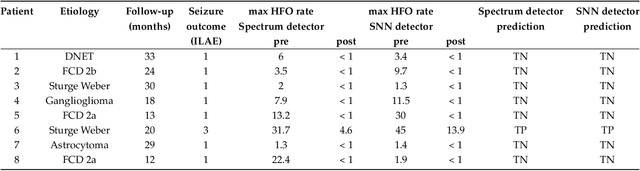
Abstract:To achieve seizure freedom, epilepsy surgery requires the complete resection of the epileptogenic brain tissue. In intraoperative ECoG recordings, high frequency oscillations (HFOs) generated by epileptogenic tissue can be used to tailor the resection margin. However, automatic detection of HFOs in real-time remains an open challenge. Here we present a spiking neural network (SNN) for automatic HFO detection that is optimally suited for neuromorphic hardware implementation. We trained the SNN to detect HFO signals measured from intraoperative ECoG on-line, using an independently labeled dataset. We targeted the detection of HFOs in the fast ripple frequency range (250-500 Hz) and compared the network results with the labeled HFO data. We endowed the SNN with a novel artifact rejection mechanism to suppress sharp transients and demonstrate its effectiveness on the ECoG dataset. The HFO rates (median 6.6 HFO/min in pre-resection recordings) detected by this SNN are comparable to those published in the dataset (58 min, 16 recordings). The postsurgical seizure outcome was "predicted" with 100% accuracy for all 8 patients. These results provide a further step towards the construction of a real-time portable battery-operated HFO detection system that can be used during epilepsy surgery to guide the resection of the epileptogenic zone.
An electronic neuromorphic system for real-time detection of High Frequency Oscillations (HFOs) in intracranial EEG
Sep 23, 2020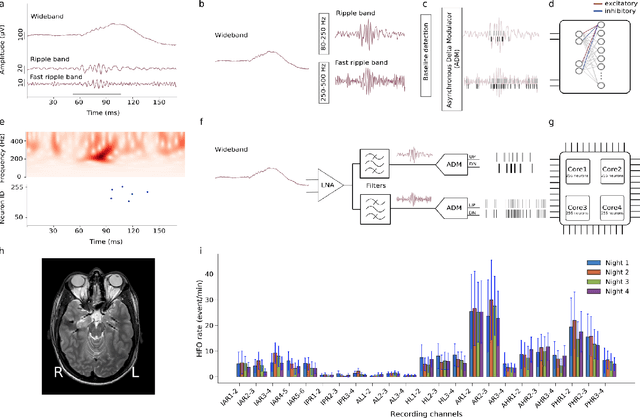

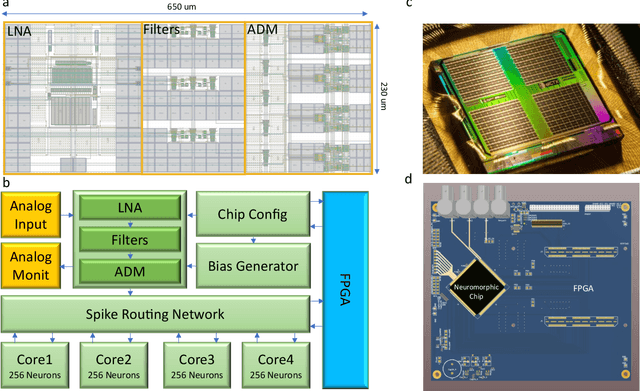

Abstract:In this work, we present a neuromorphic system that combines for the first time a neural recording headstage with a signal-to-spike conversion circuit and a multi-core spiking neural network (SNN) architecture on the same die for recording, processing, and detecting High Frequency Oscillations (HFO), which are biomarkers for the epileptogenic zone. The device was fabricated using a standard 0.18$\mu$m CMOS technology node and has a total area of 99mm$^{2}$. We demonstrate its application to HFO detection in the iEEG recorded from 9 patients with temporal lobe epilepsy who subsequently underwent epilepsy surgery. The total average power consumption of the chip during the detection task was 614.3$\mu$W. We show how the neuromorphic system can reliably detect HFOs: the system predicts postsurgical seizure outcome with state-of-the-art accuracy, specificity and sensitivity (78%, 100%, and 33% respectively). This is the first feasibility study towards identifying relevant features in intracranial human data in real-time, on-chip, using event-based processors and spiking neural networks. By providing "neuromorphic intelligence" to neural recording circuits the approach proposed will pave the way for the development of systems that can detect HFO areas directly in the operation room and improve the seizure outcome of epilepsy surgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge