Jihye Baek
Multiparametric quantification and visualization of liver fat using ultrasound
Apr 02, 2024



Abstract:Objectives- Several ultrasound measures have shown promise for assessment of steatosis compared to traditional B-scan, however clinicians may be required to integrate information across the parameters. Here, we propose an integrated multiparametric approach, enabling simple clinical assessment of key information from combined ultrasound parameters. Methods- We have measured 13 parameters related to ultrasound and shear wave elastography. These were measured in 30 human subjects under a study of liver fat. The 13 individual measures are assessed for their predictive value using independent magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) measurements as a reference standard. In addition, a comprehensive and fine-grain analysis is made of all possible combinations of sub-sets of these parameters to determine if any subset can be efficiently combined to predict fat fraction. Results- We found that as few as four key parameters related to ultrasound propagation are sufficient to generate a linear multiparametric parameter with a correlation against MRI-PDFF values of greater than 0.93. This optimal combination was found to have a classification area under the curve (AUC) approaching 1.0 when applying a threshold for separating steatosis grade zero from higher classes. Furthermore, a strategy is developed for applying local estimates of fat content as a color overlay to produce a visual impression of the extent and distribution of fat within the liver. Conclusion- In principle, this approach can be applied to most clinical ultrasound systems to provide the clinician and patient with a rapid and inexpensive estimate of liver fat content.
Improving the diagnosis of breast cancer based on biophysical ultrasound features utilizing machine learning
Jul 13, 2022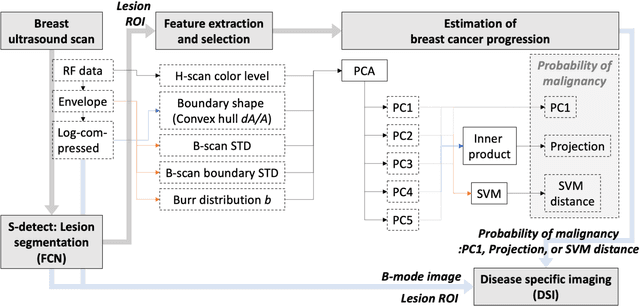

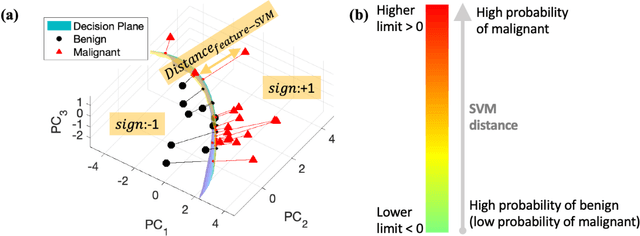
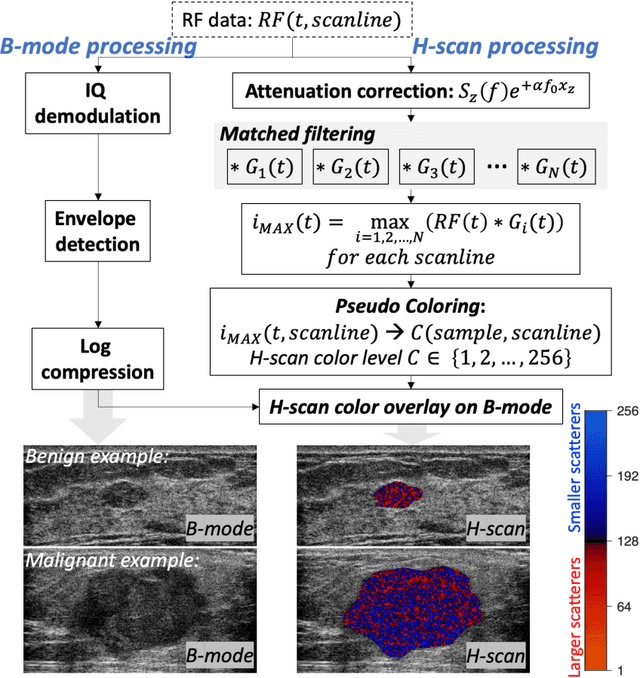
Abstract:The improved diagnostic accuracy of ultrasound breast examinations remains an important goal. In this study, we propose a biophysical feature based machine learning method for breast cancer detection to improve the performance beyond a benchmark deep learning algorithm and to furthermore provide a color overlay visual map of the probability of malignancy within a lesion. This overall framework is termed disease specific imaging. Previously, 150 breast lesions were segmented and classified utilizing a modified fully convolutional network and a modified GoogLeNet, respectively. In this study multiparametric analysis was performed within the contoured lesions. Features were extracted from ultrasound radiofrequency, envelope, and log compressed data based on biophysical and morphological models. The support vector machine with a Gaussian kernel constructed a nonlinear hyperplane, and we calculated the distance between the hyperplane and data point of each feature in multiparametric space. The distance can quantitatively assess a lesion, and suggest the probability of malignancy that is color coded and overlaid onto B mode images. Training and evaluation were performed on in vivo patient data. The overall accuracy for the most common types and sizes of breast lesions in our study exceeded 98.0% for classification and 0.98 for an area under the receiver operating characteristic curve, which is more precise than the performance of radiologists and a deep learning system. Further, the correlation between the probability and BI RADS enables a quantitative guideline to predict breast cancer. Therefore, we anticipate that the proposed framework can help radiologists achieve more accurate and convenient breast cancer classification and detection.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge