Ahmed El Kaffas
Multiparametric quantification and visualization of liver fat using ultrasound
Apr 02, 2024



Abstract:Objectives- Several ultrasound measures have shown promise for assessment of steatosis compared to traditional B-scan, however clinicians may be required to integrate information across the parameters. Here, we propose an integrated multiparametric approach, enabling simple clinical assessment of key information from combined ultrasound parameters. Methods- We have measured 13 parameters related to ultrasound and shear wave elastography. These were measured in 30 human subjects under a study of liver fat. The 13 individual measures are assessed for their predictive value using independent magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) measurements as a reference standard. In addition, a comprehensive and fine-grain analysis is made of all possible combinations of sub-sets of these parameters to determine if any subset can be efficiently combined to predict fat fraction. Results- We found that as few as four key parameters related to ultrasound propagation are sufficient to generate a linear multiparametric parameter with a correlation against MRI-PDFF values of greater than 0.93. This optimal combination was found to have a classification area under the curve (AUC) approaching 1.0 when applying a threshold for separating steatosis grade zero from higher classes. Furthermore, a strategy is developed for applying local estimates of fat content as a color overlay to produce a visual impression of the extent and distribution of fat within the liver. Conclusion- In principle, this approach can be applied to most clinical ultrasound systems to provide the clinician and patient with a rapid and inexpensive estimate of liver fat content.
Computational Enhancement of Molecularly Targeted Contrast-Enhanced Ultrasound: Application to Human Breast Tumor Imaging
Jun 22, 2020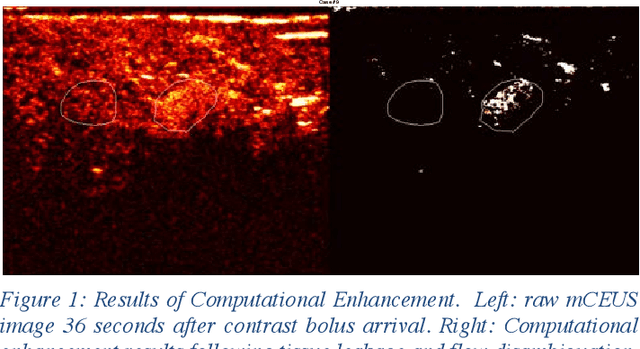
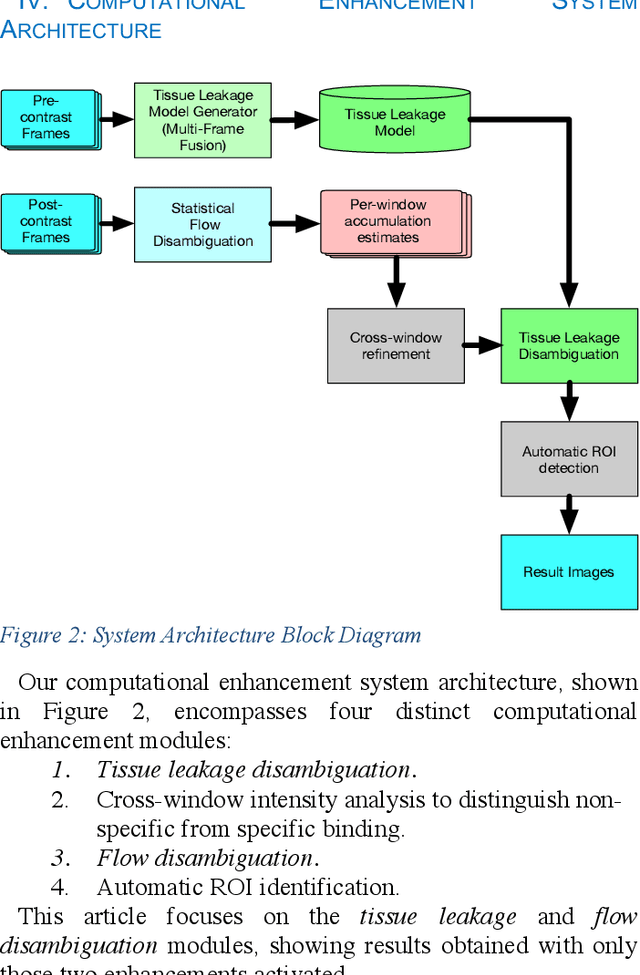
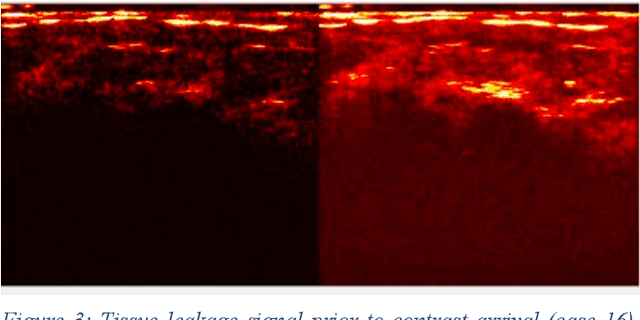

Abstract:Molecularly targeted contrast enhanced ultrasound (mCEUS) is a clinically promising approach for early cancer detection through targeted imaging of VEGFR2 (KDR) receptors. We have developed computational enhancement techniques for mCEUS tailored to address the unique challenges of imaging contrast accumulation in humans. These techniques utilize dynamic analysis to distinguish molecularly bound contrast agent from other contrast-mode signal sources, enabling analysis of contrast agent accumulation to be performed during contrast bolus arrival when the signal due to molecular binding is strongest. Applied to the 18 human patient examinations of the first-in-human molecular ultrasound breast lesion study, computational enhancement improved the ability to differentiate between pathology-proven lesion and pathology-proven normal tissue in real-world human examination conditions that involved both patient and probe motion, with improvements in contrast ratio between lesion and normal tissue that in most cases exceed an order of magnitude (10x). Notably, computational enhancement eliminated a false positive result in which tissue leakage signal was misinterpreted by radiologists to be contrast agent accumulation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge