Ioannis E. Polykretis
Introducing Astrocytes on a Neuromorphic Processor: Synchronization, Local Plasticity and Edge of Chaos
Jul 02, 2019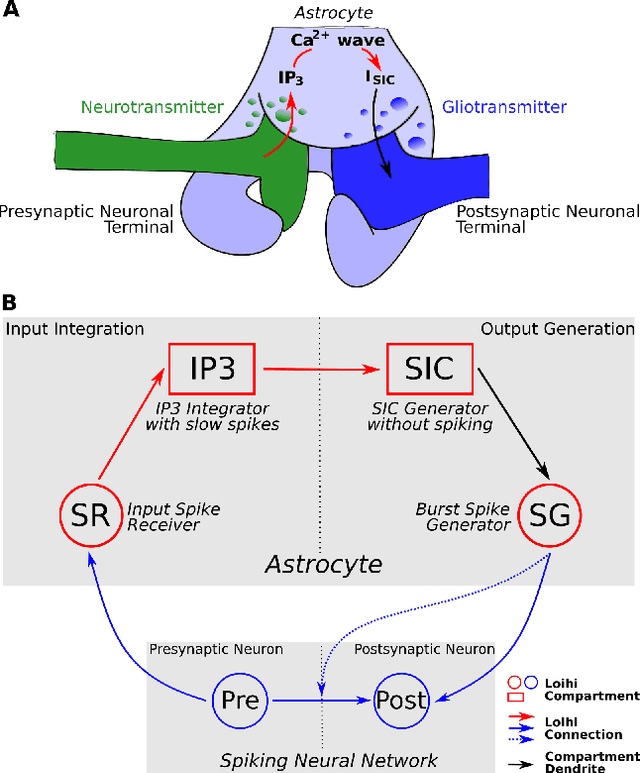
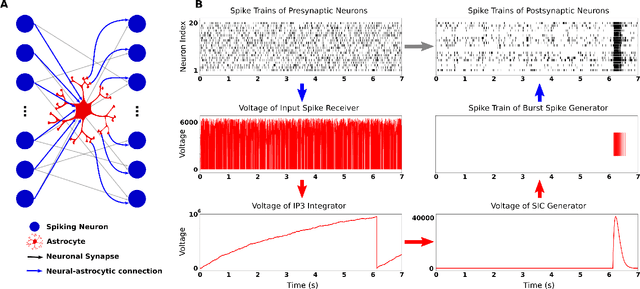


Abstract:While there is still a lot to learn about astrocytes and their neuromodulatory role associated with their spatial and temporal integration of synaptic activity, the introduction of an additional to neurons processing unit into neuromorphic hardware is timely, facilitating their computational exploration in basic science questions and their exploitation in real-world applications. Here, we present an astrocytic module that enables the development of a spiking Neuronal-Astrocytic Network (SNAN) into Intel's Loihi neuromorphic chip. The basis of our module is an end-to-end biophysically plausible compartmental model of an astrocyte that simulates how intracellular activity may encode synaptic activity in space and time. To demonstrate the functional role of astrocytes in SNANs, we describe how an astrocyte may sense and induce activity-dependent neuronal synchronization, can endow single-shot learning capabilities in spike-time-dependent plasticity (STDP), and sense the transition between ordered and chaotic activity in the neuronal component of an SNAN. Our astrocytic module may serve as a natural extension for neuromorphic hardware by mimicking the distinct computational roles of its biological counterpart.
Axonal Conduction Velocity Impacts Neuronal Network Oscillations
Mar 22, 2019

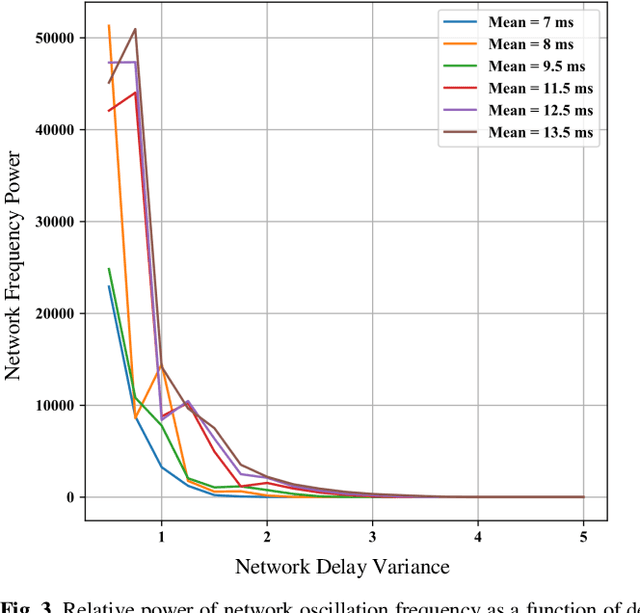

Abstract:Increasing experimental evidence suggests that axonal action potential conduction velocity is a highly adaptive parameter in the adult central nervous system. Yet, the effects of this newfound plasticity on global brain dynamics is poorly understood. In this work, we analyzed oscillations in biologically plausible neuronal networks with different conduction velocity distributions. Changes of 1-2 (ms) in network mean signal transmission time resulted in substantial network oscillation frequency changes ranging in 0-120 (Hz). Our results suggest that changes in axonal conduction velocity may significantly affect both the frequency and synchrony of brain rhythms, which have well established connections to learning, memory, and other cognitive processes.
Computational Astrocyence: Astrocytes encode inhibitory activity into the frequency and spatial extent of their calcium elevations
Mar 18, 2019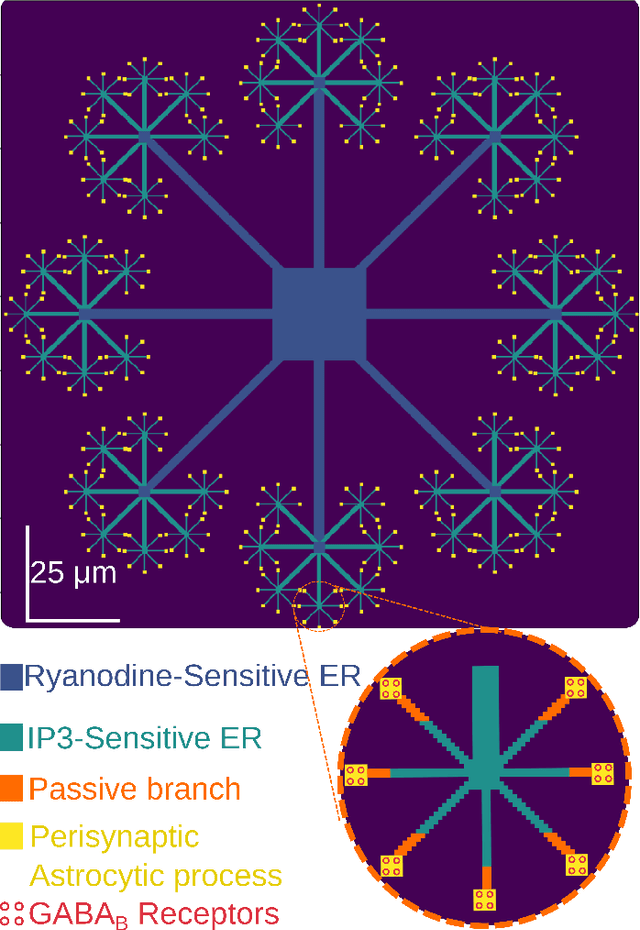
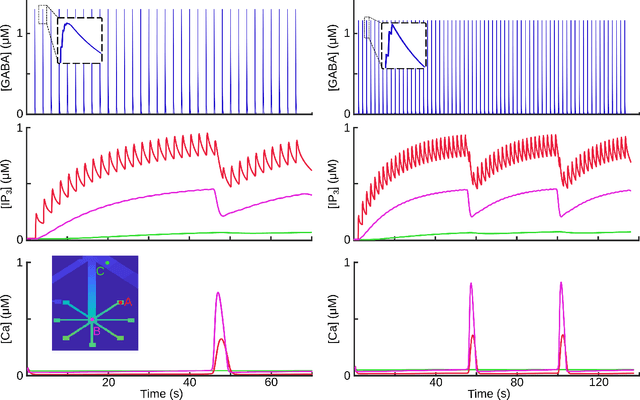
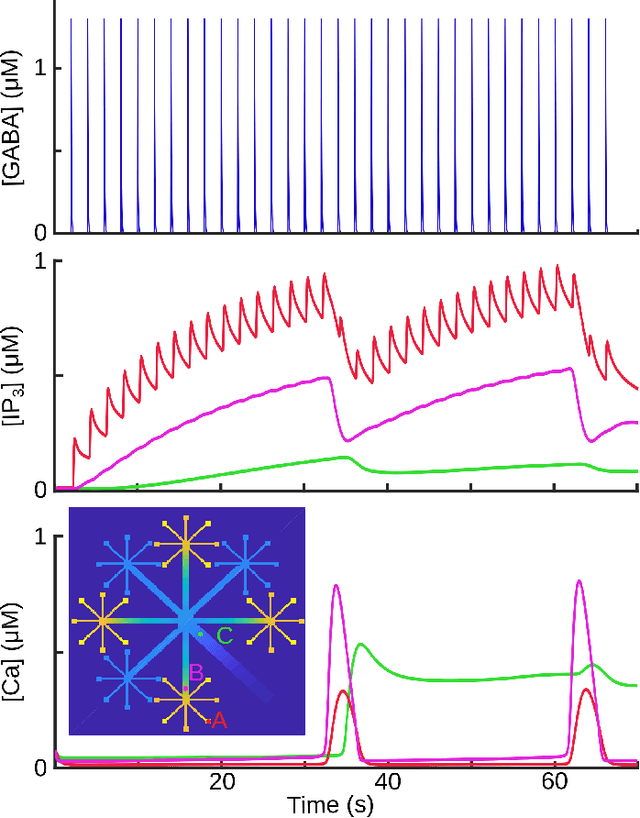
Abstract:Deciphering the complex interactions between neurotransmission and astrocytic $Ca^{2+}$ elevations is a target promising a comprehensive understanding of brain function. While the astrocytic response to excitatory synaptic activity has been extensively studied, how inhibitory activity results to intracellular $Ca^{2+}$ waves remains elusive. In this study, we developed a compartmental astrocytic model that exhibits distinct levels of responsiveness to inhibitory activity. Our model suggested that the astrocytic coverage of inhibitory terminals defines the spatial and temporal scale of their $Ca^{2+}$ elevations. Understanding the interplay between the synaptic pathways and the astrocytic responses will help us identify how astrocytes work independently and cooperatively with neurons, in health and disease.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge