Igor Tetko
SEISMO: Increasing Sample Efficiency in Molecular Optimization with a Trajectory-Aware LLM Agent
Jan 31, 2026Abstract:Optimizing the structure of molecules to achieve desired properties is a central bottleneck across the chemical sciences, particularly in the pharmaceutical industry where it underlies the discovery of new drugs. Since molecular property evaluation often relies on costly and rate-limited oracles, such as experimental assays, molecular optimization must be highly sample-efficient. To address this, we introduce SEISMO, an LLM agent that performs strictly online, inference-time molecular optimization, updating after every oracle call without the need for population-based or batched learning. SEISMO conditions each proposal on the full optimization trajectory, combining natural-language task descriptions with scalar scores and, when available, structured explanatory feedback. Across the Practical Molecular Optimization benchmark of 23 tasks, SEISMO achieves a 2-3 times higher area under the optimisation curve than prior methods, often reaching near-maximal task scores within 50 oracle calls. Our additional medicinal-chemistry tasks show that providing explanatory feedback further improves efficiency, demonstrating that leveraging domain knowledge and structured information is key to sample-efficient molecular optimization.
Models Matter: The Impact of Single-Step Retrosynthesis on Synthesis Planning
Aug 10, 2023Abstract:Retrosynthesis consists of breaking down a chemical compound recursively step-by-step into molecular precursors until a set of commercially available molecules is found with the goal to provide a synthesis route. Its two primary research directions, single-step retrosynthesis prediction, which models the chemical reaction logic, and multi-step synthesis planning, which tries to find the correct sequence of reactions, are inherently intertwined. Still, this connection is not reflected in contemporary research. In this work, we combine these two major research directions by applying multiple single-step retrosynthesis models within multi-step synthesis planning and analyzing their impact using public and proprietary reaction data. We find a disconnection between high single-step performance and potential route-finding success, suggesting that single-step models must be evaluated within synthesis planning in the future. Furthermore, we show that the commonly used single-step retrosynthesis benchmark dataset USPTO-50k is insufficient as this evaluation task does not represent model performance and scalability on larger and more diverse datasets. For multi-step synthesis planning, we show that the choice of the single-step model can improve the overall success rate of synthesis planning by up to +28% compared to the commonly used baseline model. Finally, we show that each single-step model finds unique synthesis routes, and differs in aspects such as route-finding success, the number of found synthesis routes, and chemical validity, making the combination of single-step retrosynthesis prediction and multi-step synthesis planning a crucial aspect when developing future methods.
Mind the Retrosynthesis Gap: Bridging the divide between Single-step and Multi-step Retrosynthesis Prediction
Dec 12, 2022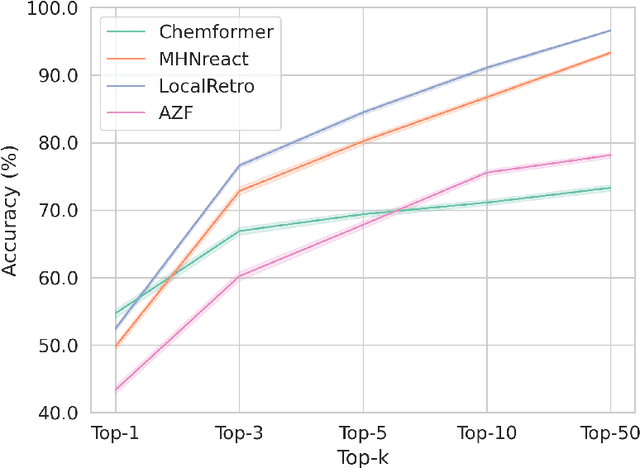
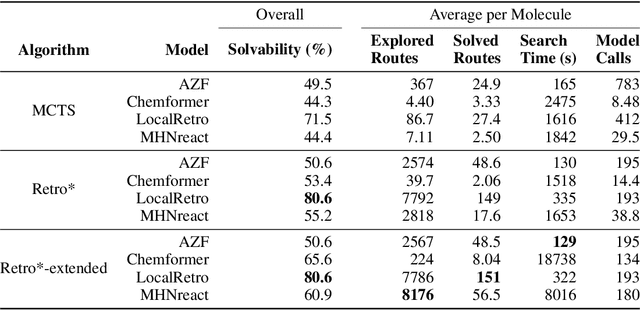
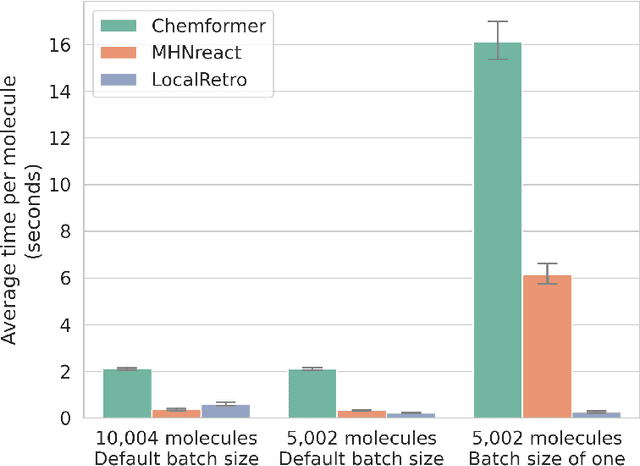
Abstract:Retrosynthesis is the task of breaking down a chemical compound recursively step-by-step into molecular precursors until a set of commercially available molecules is found. Consequently, the goal is to provide a valid synthesis route for a molecule. As more single-step models develop, we see increasing accuracy in the prediction of molecular disconnections, potentially improving the creation of synthetic paths. Multi-step approaches repeatedly apply the chemical information stored in single-step retrosynthesis models. However, this connection is not reflected in contemporary research, fixing either the single-step model or the multi-step algorithm in the process. In this work, we establish a bridge between both tasks by benchmarking the performance and transfer of different single-step retrosynthesis models to the multi-step domain by leveraging two common search algorithms, Monte Carlo Tree Search and Retro*. We show that models designed for single-step retrosynthesis, when extended to multi-step, can have a tremendous impact on the route finding capabilities of current multi-step methods, improving performance by up to +30% compared to the most widely used model. Furthermore, we observe no clear link between contemporary single-step and multi-step evaluation metrics, showing that single-step models need to be developed and tested for the multi-step domain and not as an isolated task to find synthesis routes for molecules of interest.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge