Hayato Idei
Sensory attenuation develops as a result of sensorimotor experience
Dec 01, 2021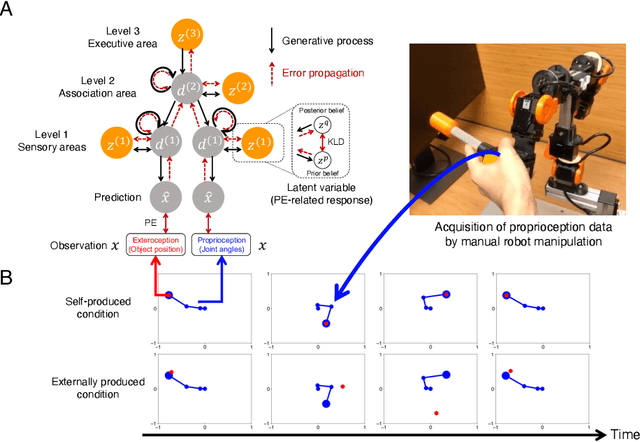
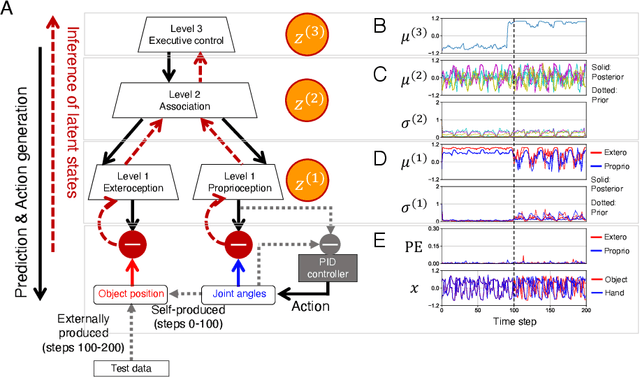
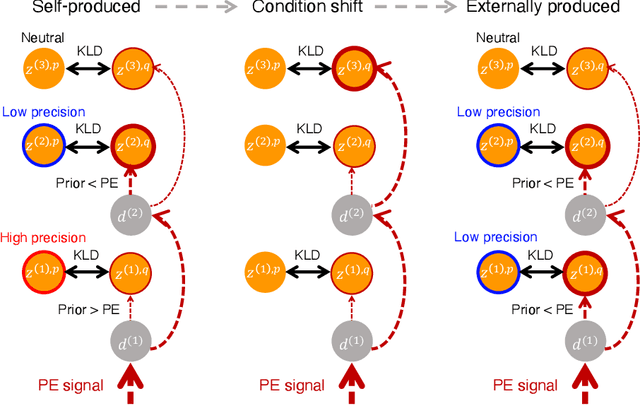
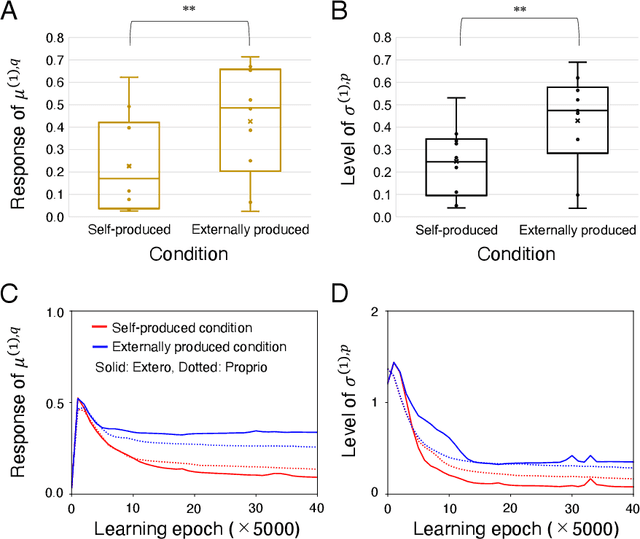
Abstract:The brain attenuates its responses to self-produced exteroceptions (e.g., we cannot tickle ourselves). Is this phenomenon, known as sensory attenuation, enabled innately, or is it acquired through learning? For decades, theoretical and biological studies have suggested related neural functions of sensory attenuation, such as an efference copy of the motor command and neuromodulation; however, the developmental aspect of sensory attenuation remains unexamined. Here, our simulation study using a recurrent neural network, operated according to a computational principle called free-energy minimization, shows that sensory attenuation can be developed as a free-energy state in the network through learning of two distinct types of sensorimotor patterns, characterized by self-produced or externally produced exteroceptive feedback. Simulation of the network, consisting of sensory (proprioceptive and exteroceptive), association, and executive areas, showed that shifts between these two types of sensorimotor patterns triggered transitions from one free-energy state to another in the network. Consequently, this induced shifts between attenuating and amplifying responses in the sensory areas. Furthermore, the executive area, proactively adjusted the precision of the prediction in lower levels while being modulated by the bottom-up sensory prediction error signal in minimizing the free-energy, thereby serving as an information hub in generating the observed shifts. We also found that innate alterations in modulation of sensory-information flow induced some characteristics analogous to schizophrenia and autism spectrum disorder. This study provides a novel perspective on neural mechanisms underlying emergence of perceptual phenomena and psychiatric disorders.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge