Harris Vince
Investigating Deep-Learning NLP for Automating the Extraction of Oncology Efficacy Endpoints from Scientific Literature
Nov 03, 2023



Abstract:Benchmarking drug efficacy is a critical step in clinical trial design and planning. The challenge is that much of the data on efficacy endpoints is stored in scientific papers in free text form, so extraction of such data is currently a largely manual task. Our objective is to automate this task as much as possible. In this study we have developed and optimised a framework to extract efficacy endpoints from text in scientific papers, using a machine learning approach. Our machine learning model predicts 25 classes associated with efficacy endpoints and leads to high F1 scores (harmonic mean of precision and recall) of 96.4% on the test set, and 93.9% and 93.7% on two case studies. These methods were evaluated against - and showed strong agreement with - subject matter experts and show significant promise in the future of automating the extraction of clinical endpoints from free text. Clinical information extraction from text data is currently a laborious manual task which scales poorly and is prone to human error. Demonstrating the ability to extract efficacy endpoints automatically shows great promise for accelerating clinical trial design moving forwards.
Towards Fully Automated Segmentation of Rat Cardiac MRI by Leveraging Deep Learning Frameworks
Sep 09, 2021
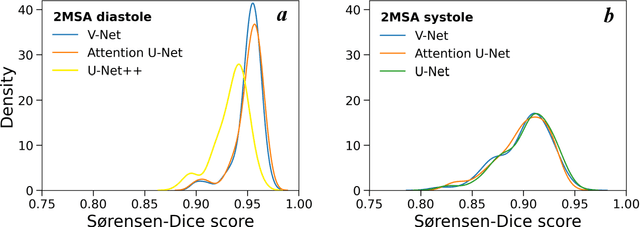


Abstract:Automated segmentation of human cardiac magnetic resonance datasets has been steadily improving during recent years. However, these methods are not directly applicable in preclinical context due to limited datasets and lower image resolution. Successful application of deep architectures for rat cardiac segmentation, although of critical importance for preclinical evaluation of cardiac function, has to our knowledge not yet been reported. We developed segmentation models that expand on the standard U-Net architecture and evaluated separate models for systole and diastole phases, 2MSA, and one model for all timepoints, 1MSA. Furthermore, we calibrated model outputs using a Gaussian Process (GP)-based prior to improve phase selection. Resulting models approach human performance in terms of left ventricular segmentation quality and ejection fraction (EF) estimation in both 1MSA and 2MSA settings (S{\o}rensen-Dice score 0.91 +/- 0.072 and 0.93 +/- 0.032, respectively). 2MSA achieved a mean absolute difference between estimated and reference EF of 3.5 +/- 2.5 %, while 1MSA resulted in 4.1 +/- 3.0 %. Applying Gaussian Processes to 1MSA allows to automate the selection of systole and diastole phases. Combined with a novel cardiac phase selection strategy, our work presents an important first step towards a fully automated segmentation pipeline in the context of rat cardiac analysis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge