Govind Makharia
MeasureNet: Measurement Based Celiac Disease Identification
Dec 02, 2024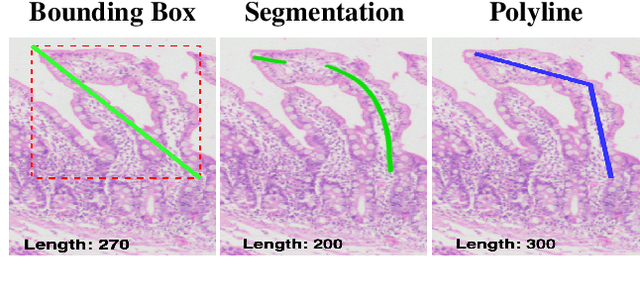
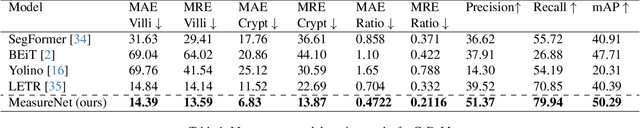
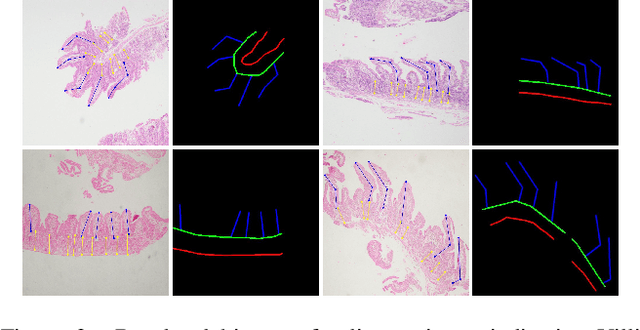
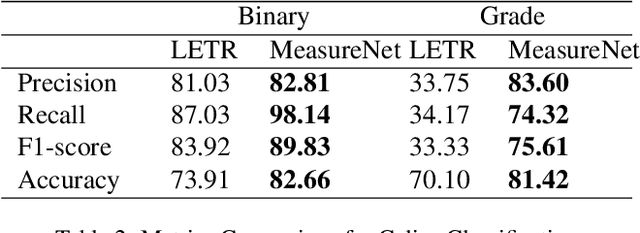
Abstract:Celiac disease is an autoimmune disorder triggered by the consumption of gluten. It causes damage to the villi, the finger-like projections in the small intestine that are responsible for nutrient absorption. Additionally, the crypts, which form the base of the villi, are also affected, impairing the regenerative process. The deterioration in villi length, computed as the villi-to-crypt length ratio, indicates the severity of celiac disease. However, manual measurement of villi-crypt length can be both time-consuming and susceptible to inter-observer variability, leading to inconsistencies in diagnosis. While some methods can perform measurement as a post-hoc process, they are prone to errors in the initial stages. This gap underscores the need for pathologically driven solutions that enhance measurement accuracy and reduce human error in celiac disease assessments. Our proposed method, MeasureNet, is a pathologically driven polyline detection framework incorporating polyline localization and object-driven losses specifically designed for measurement tasks. Furthermore, we leverage segmentation model to provide auxiliary guidance about crypt location when crypt are partially visible. To ensure that model is not overdependent on segmentation mask we enhance model robustness through a mask feature mixup technique. Additionally, we introduce a novel dataset for grading celiac disease, consisting of 750 annotated duodenum biopsy images. MeasureNet achieves an 82.66% classification accuracy for binary classification and 81% accuracy for multi-class grading of celiac disease. Code: https://github.com/dair-iitd/MeasureNet
DeGPR: Deep Guided Posterior Regularization for Multi-Class Cell Detection and Counting
Apr 03, 2023Abstract:Multi-class cell detection and counting is an essential task for many pathological diagnoses. Manual counting is tedious and often leads to inter-observer variations among pathologists. While there exist multiple, general-purpose, deep learning-based object detection and counting methods, they may not readily transfer to detecting and counting cells in medical images, due to the limited data, presence of tiny overlapping objects, multiple cell types, severe class-imbalance, minute differences in size/shape of cells, etc. In response, we propose guided posterior regularization (DeGPR), which assists an object detector by guiding it to exploit discriminative features among cells. The features may be pathologist-provided or inferred directly from visual data. We validate our model on two publicly available datasets (CoNSeP and MoNuSAC), and on MuCeD, a novel dataset that we contribute. MuCeD consists of 55 biopsy images of the human duodenum for predicting celiac disease. We perform extensive experimentation with three object detection baselines on three datasets to show that DeGPR is model-agnostic, and consistently improves baselines obtaining up to 9% (absolute) mAP gains.
Contrastive Semi-Supervised Learning for 2D Medical Image Segmentation
Jul 10, 2021



Abstract:Contrastive Learning (CL) is a recent representation learning approach, which encourages inter-class separability and intra-class compactness in learned image representations. Since medical images often contain multiple semantic classes in an image, using CL to learn representations of local features (as opposed to global) is important. In this work, we present a novel semi-supervised 2D medical segmentation solution that applies CL on image patches, instead of full images. These patches are meaningfully constructed using the semantic information of different classes obtained via pseudo labeling. We also propose a novel consistency regularization (CR) scheme, which works in synergy with CL. It addresses the problem of confirmation bias, and encourages better clustering in the feature space. We evaluate our method on four public medical segmentation datasets and a novel histopathology dataset that we introduce. Our method obtains consistent improvements over state-of-the-art semi-supervised segmentation approaches for all datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge