Filip Stojceski
LightCPPgen: An Explainable Machine Learning Pipeline for Rational Design of Cell Penetrating Peptides
May 31, 2024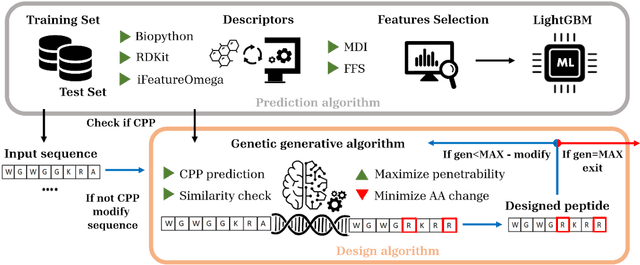
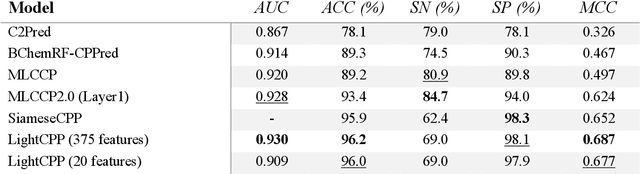
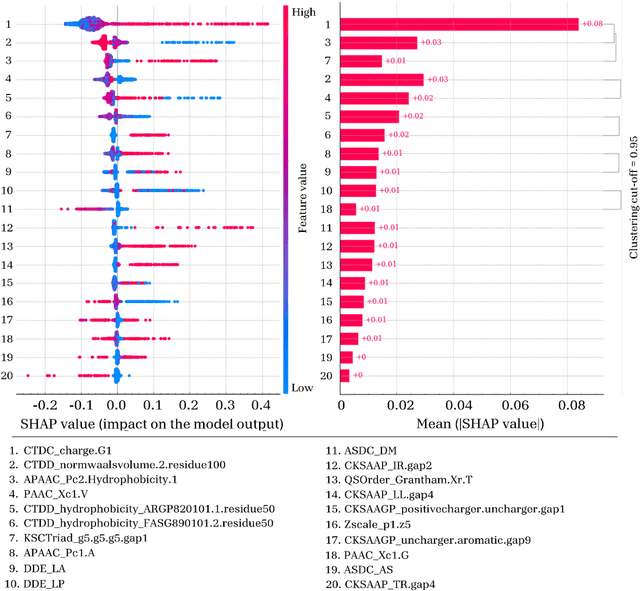
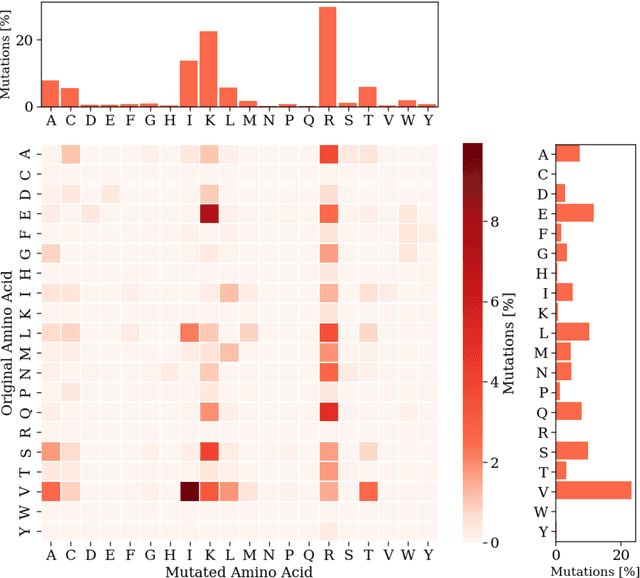
Abstract:Cell-penetrating peptides (CPPs) are powerful vectors for the intracellular delivery of a diverse array of therapeutic molecules. Despite their potential, the rational design of CPPs remains a challenging task that often requires extensive experimental efforts and iterations. In this study, we introduce an innovative approach for the de novo design of CPPs, leveraging the strengths of machine learning (ML) and optimization algorithms. Our strategy, named LightCPPgen, integrates a LightGBM-based predictive model with a genetic algorithm (GA), enabling the systematic generation and optimization of CPP sequences. At the core of our methodology is the development of an accurate, efficient, and interpretable predictive model, which utilizes 20 explainable features to shed light on the critical factors influencing CPP translocation capacity. The CPP predictive model works synergistically with an optimization algorithm, which is tuned to enhance computational efficiency while maintaining optimization performance. The GA solutions specifically target the candidate sequences' penetrability score, while trying to maximize similarity with the original non-penetrating peptide in order to retain its original biological and physicochemical properties. By prioritizing the synthesis of only the most promising CPP candidates, LightCPPgen can drastically reduce the time and cost associated with wet lab experiments. In summary, our research makes a substantial contribution to the field of CPP design, offering a robust framework that combines ML and optimization techniques to facilitate the rational design of penetrating peptides, by enhancing the explainability and interpretability of the design process.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge