Faik C. Meral
Dehazing Ultrasound using Diffusion Models
Jul 20, 2023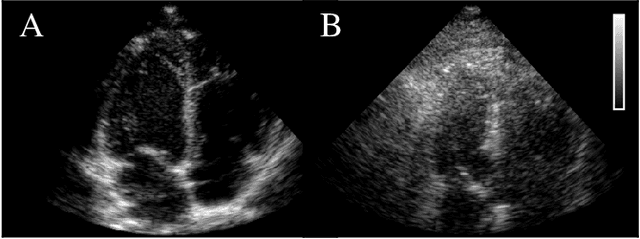
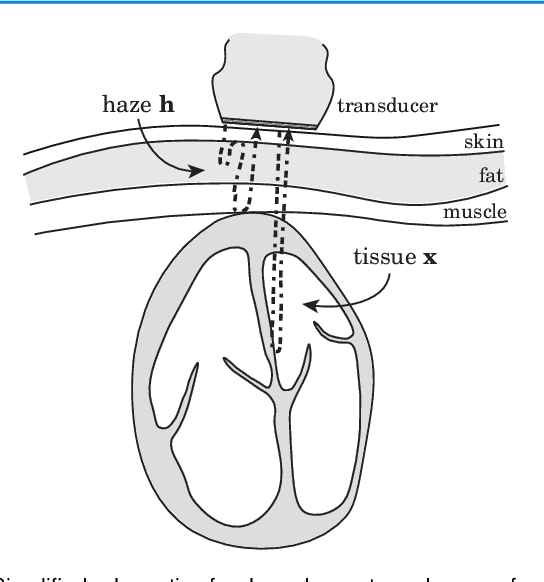
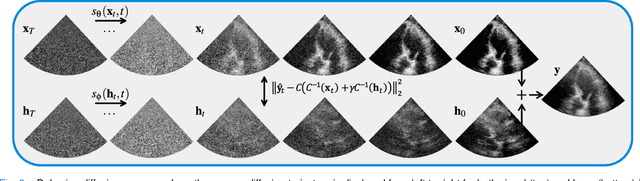
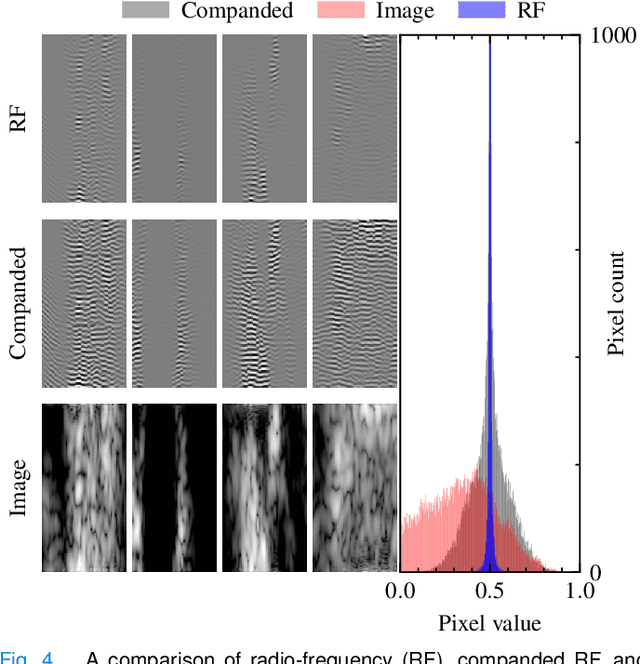
Abstract:Echocardiography has been a prominent tool for the diagnosis of cardiac disease. However, these diagnoses can be heavily impeded by poor image quality. Acoustic clutter emerges due to multipath reflections imposed by layers of skin, subcutaneous fat, and intercostal muscle between the transducer and heart. As a result, haze and other noise artifacts pose a real challenge to cardiac ultrasound imaging. In many cases, especially with difficult-to-image patients such as patients with obesity, a diagnosis from B-Mode ultrasound imaging is effectively rendered unusable, forcing sonographers to resort to contrast-enhanced ultrasound examinations or refer patients to other imaging modalities. Tissue harmonic imaging has been a popular approach to combat haze, but in severe cases is still heavily impacted by haze. Alternatively, denoising algorithms are typically unable to remove highly structured and correlated noise, such as haze. It remains a challenge to accurately describe the statistical properties of structured haze, and develop an inference method to subsequently remove it. Diffusion models have emerged as powerful generative models and have shown their effectiveness in a variety of inverse problems. In this work, we present a joint posterior sampling framework that combines two separate diffusion models to model the distribution of both clean ultrasound and haze in an unsupervised manner. Furthermore, we demonstrate techniques for effectively training diffusion models on radio-frequency ultrasound data and highlight the advantages over image data. Experiments on both \emph{in-vitro} and \emph{in-vivo} cardiac datasets show that the proposed dehazing method effectively removes haze while preserving signals from weakly reflected tissue.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge