Elhoucine Elfatimi
Model Checking in Medical Imaging for Tumor Detection and Segmentation
Jan 07, 2025


Abstract:Recent advancements in model checking have demonstrated significant potential across diverse applications, particularly in signal and image analysis. Medical imaging stands out as a critical domain where model checking can be effectively applied to design and evaluate robust frameworks. These frameworks facilitate automatic and semi-automatic delineation of regions of interest within images, aiding in accurate segmentation. This paper provides a comprehensive analysis of recent works leveraging spatial logic to develop operators and tools for identifying regions of interest, including tumorous and non-tumorous areas. Additionally, we examine the challenges inherent to spatial model-checking techniques, such as variability in ground truth data and the need for streamlined procedures suitable for routine clinical practice.
A Hybrid Deep Learning and Model-Checking Framework for Accurate Brain Tumor Detection and Validation
Dec 31, 2024Abstract:Model checking, a formal verification technique, ensures systems meet predefined requirements, playing a crucial role in minimizing errors and enhancing quality during development. This paper introduces a novel hybrid framework integrating model checking with deep learning for brain tumor detection and validation in medical imaging. By combining model-checking principles with CNN-based feature extraction and K-FCM clustering for segmentation, the proposed approach enhances the reliability of tumor detection and segmentation. Experimental results highlight the framework's effectiveness, achieving 98\% accuracy, 96.15\% precision, and 100\% recall, demonstrating its potential as a robust tool for advanced medical image analysis.
Uncertainty Quantified Deep Learning and Regression Analysis Framework for Image Segmentation of Skin Cancer Lesions
Dec 28, 2024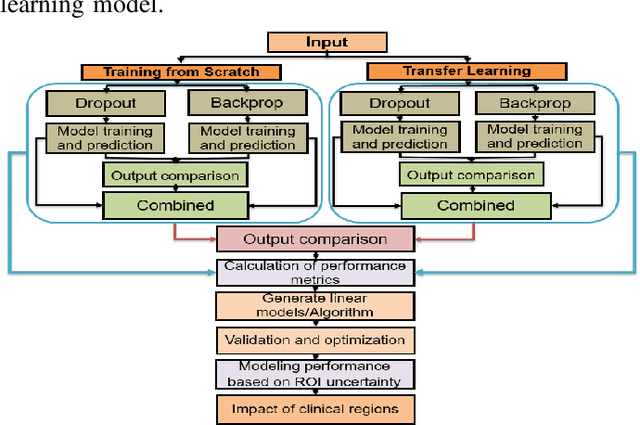
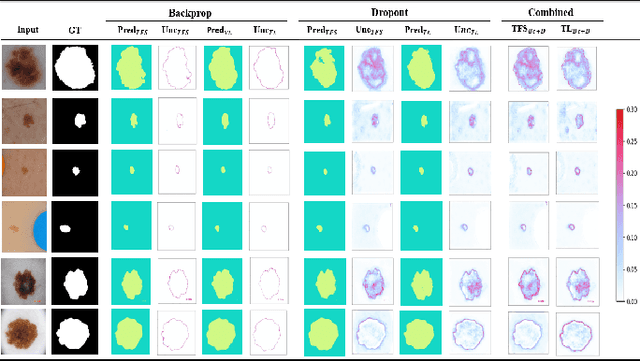
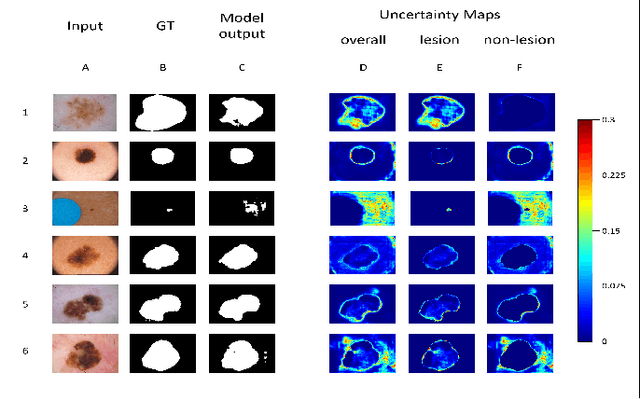
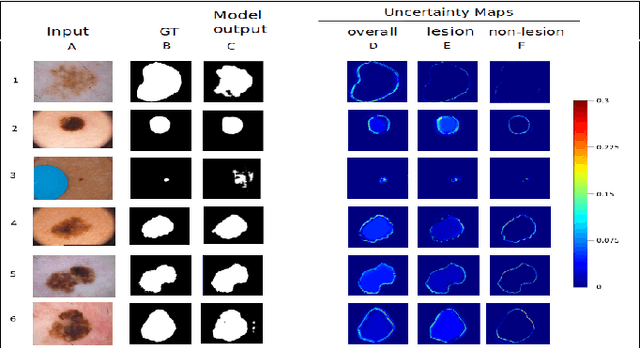
Abstract:Deep learning models (DLMs) frequently achieve accurate segmentation and classification of tumors from medical images. However, DLMs lacking feedback on their image segmentation mechanisms, such as Dice coefficients and confidence in their performance, face challenges when processing previously unseen images in real-world clinical settings. Uncertainty estimates to identify DLM predictions at the cellular or single-pixel level that require clinician review can enhance trust. However, their deployment requires significant computational resources. This study reports two DLMs, one trained from scratch and another based on transfer learning, with Monte Carlo dropout or Bayes-by-backprop uncertainty estimations to segment lesions from the publicly available The International Skin Imaging Collaboration-19 dermoscopy image database with cancerous lesions. A novel approach to compute pixel-by-pixel uncertainty estimations of DLM segmentation performance in multiple clinical regions from a single dermoscopy image with corresponding Dice scores is reported for the first time. Image-level uncertainty maps demonstrated correspondence between imperfect DLM segmentation and high uncertainty levels in specific skin tissue regions, with or without lesions. Four new linear regression models that can predict the Dice performance of DLM segmentation using constants and uncertainty measures, either individually or in combination from lesions, tissue structures, and non-tissue pixel regions critical for clinical diagnosis and prognostication in skin images (Spearman's correlation, p < 0.05), are reported for the first time for low-compute uncertainty estimation workflows.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge