Dominik Klepl
Graph Neural Network-based EEG Classification: A Survey
Oct 03, 2023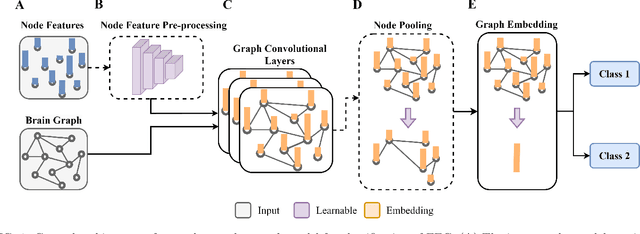
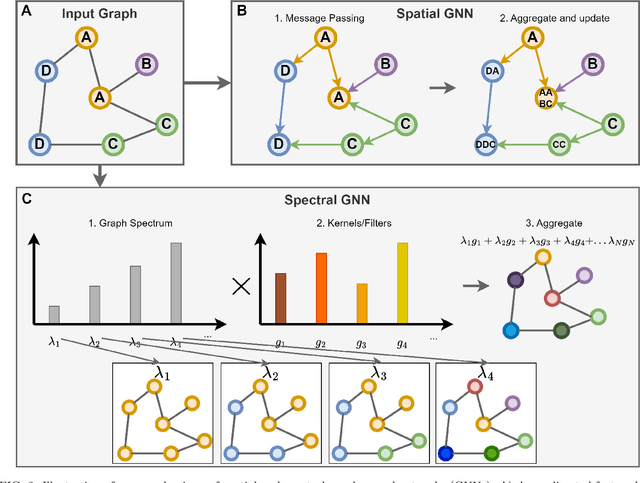
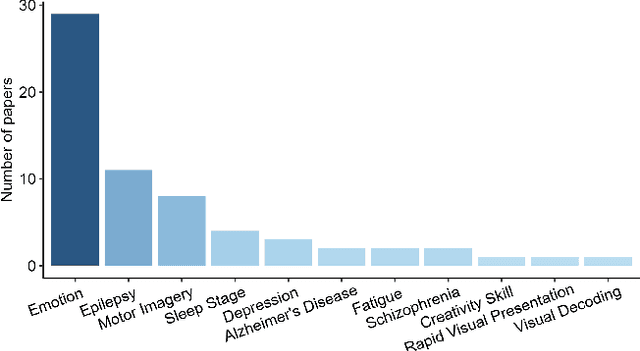
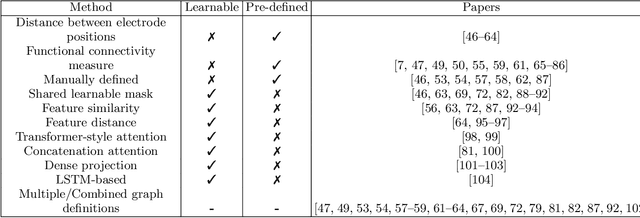
Abstract:Graph neural networks (GNN) are increasingly used to classify EEG for tasks such as emotion recognition, motor imagery and neurological diseases and disorders. A wide range of methods have been proposed to design GNN-based classifiers. Therefore, there is a need for a systematic review and categorisation of these approaches. We exhaustively search the published literature on this topic and derive several categories for comparison. These categories highlight the similarities and differences among the methods. The results suggest a prevalence of spectral graph convolutional layers over spatial. Additionally, we identify standard forms of node features, with the most popular being the raw EEG signal and differential entropy. Our results summarise the emerging trends in GNN-based approaches for EEG classification. Finally, we discuss several promising research directions, such as exploring the potential of transfer learning methods and appropriate modelling of cross-frequency interactions.
Adaptive Gated Graph Convolutional Network for Explainable Diagnosis of Alzheimer's Disease using EEG Data
Apr 12, 2023



Abstract:Graph neural network (GNN) models are increasingly being used for the classification of electroencephalography (EEG) data. However, GNN-based diagnosis of neurological disorders, such as Alzheimer's disease (AD), remains a relatively unexplored area of research. Previous studies have relied on functional connectivity methods to infer brain graph structures and used simple GNN architectures for the diagnosis of AD. In this work, we propose a novel adaptive gated graph convolutional network (AGGCN) that can provide explainable predictions. AGGCN adaptively learns graph structures by combining convolution-based node feature enhancement with a well-known correlation-based measure of functional connectivity. Furthermore, the gated graph convolution can dynamically weigh the contribution of various spatial scales. The proposed model achieves high accuracy in both eyes-closed and eyes-open conditions, indicating the stability of learned representations. Finally, we demonstrate that the proposed AGGCN model generates consistent explanations of its predictions that might be relevant for further study of AD-related alterations of brain networks.
Bispectrum-based Cross-frequency Functional Connectivity: Classification of Alzheimer's disease
Jun 10, 2022
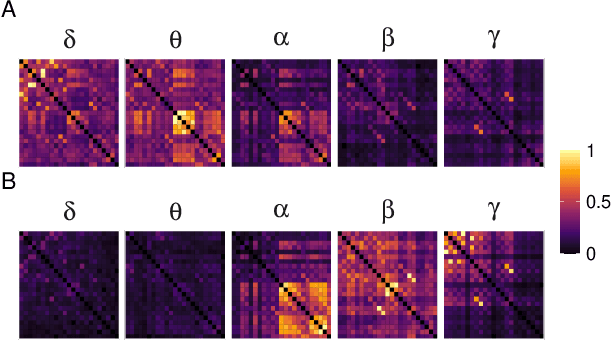
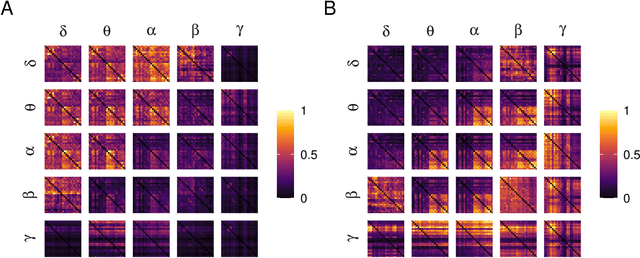
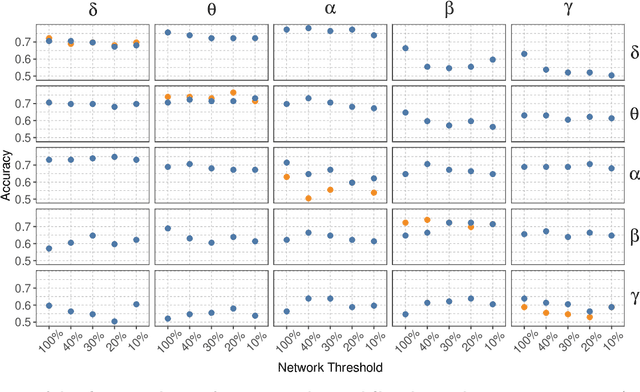
Abstract:Alzheimer's disease (AD) is a neurodegenerative disease known to affect brain functional connectivity (FC). Linear FC measures have been applied to study the differences in AD by splitting neurophysiological signals such as electroencephalography (EEG) recordings into discrete frequency bands and analysing them in isolation. We address this limitation by quantifying cross-frequency FC in addition to the traditional within-band approach. Cross-bispectrum, a higher-order spectral analysis, is used to measure the nonlinear FC and is compared with the cross-spectrum, which only measures the linear FC within bands. Each frequency coupling is then used to construct an FC network, which is in turn vectorised and used to train a classifier. We show that fusing features from networks improves classification accuracy. Although both within-frequency and cross-frequency networks can be used to predict AD with high accuracy, our results show that bispectrum-based FC outperforms cross-spectrum suggesting an important role of cross-frequency FC.
Characterising Alzheimer's Disease with EEG-based Energy Landscape Analysis
Feb 19, 2021



Abstract:Alzheimer's disease (AD) is one of the most common neurodegenerative diseases, with around 50 million patients worldwide. Accessible and non-invasive methods of diagnosing and characterising AD are therefore urgently required. Electroencephalography (EEG) fulfils these criteria and is often used when studying AD. Several features derived from EEG were shown to predict AD with high accuracy, e.g. signal complexity and synchronisation. However, the dynamics of how the brain transitions between stable states have not been properly studied in the case of AD and EEG data. Energy landscape analysis is a method that can be used to quantify these dynamics. This work presents the first application of this method to both AD and EEG. Energy landscape assigns energy value to each possible state, i.e. pattern of activations across brain regions. The energy is inversely proportional to the probability of occurrence. By studying the features of energy landscapes of 20 AD patients and 20 healthy age-matched counterparts, significant differences were found. The dynamics of AD patients' brain networks were shown to be more constrained - with more local minima, less variation in basin size, and smaller basins. We show that energy landscapes can predict AD with high accuracy, performing significantly better than baseline models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge