Deniz Sezin Ayvaz
Measuring Fairness in Financial Transaction Machine Learning Models
Jan 18, 2025



Abstract:Mastercard, a global leader in financial services, develops and deploys machine learning models aimed at optimizing card usage and preventing attrition through advanced predictive models. These models use aggregated and anonymized card usage patterns, including cross-border transactions and industry-specific spending, to tailor bank offerings and maximize revenue opportunities. Mastercard has established an AI Governance program, based on its Data and Tech Responsibility Principles, to evaluate any built and bought AI for efficacy, fairness, and transparency. As part of this effort, Mastercard has sought expertise from the Turing Institute through a Data Study Group to better assess fairness in more complex AI/ML models. The Data Study Group challenge lies in defining, measuring, and mitigating fairness in these predictions, which can be complex due to the various interpretations of fairness, gaps in the research literature, and ML-operations challenges.
Investigating Conversion from Mild Cognitive Impairment to Alzheimer's Disease using Latent Space Manipulation
Nov 16, 2021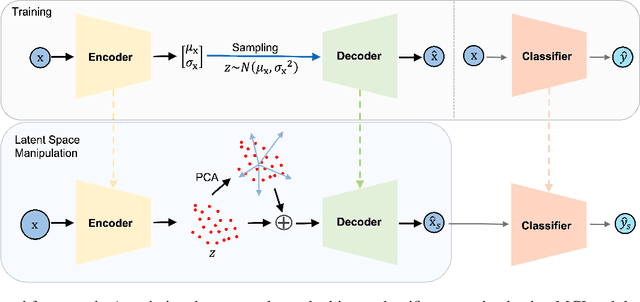
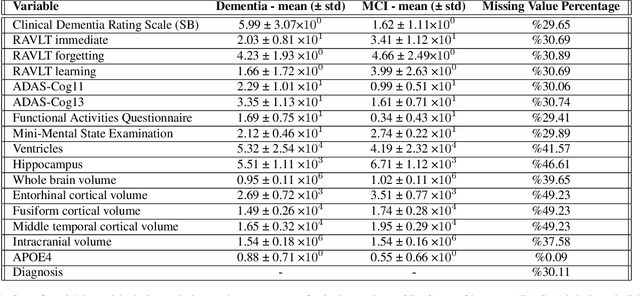
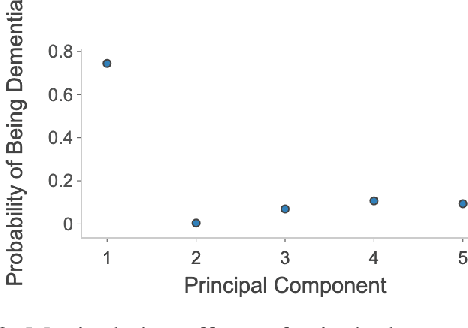
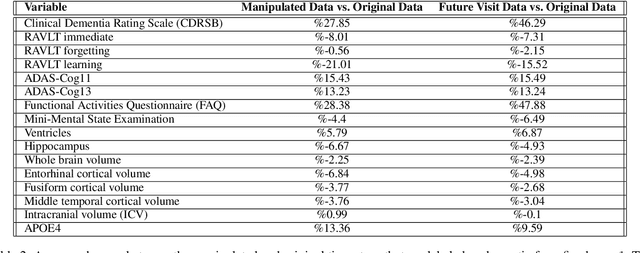
Abstract:Alzheimer's disease is the most common cause of dementia that affects millions of lives worldwide. Investigating the underlying causes and risk factors of Alzheimer's disease is essential to prevent its progression. Mild Cognitive Impairment (MCI) is considered an intermediate stage before Alzheimer's disease. Early prediction of the conversion from the MCI to Alzheimer's is crucial to take necessary precautions for decelerating the progression and developing suitable treatments. In this study, we propose a deep learning framework to discover the variables which are identifiers of the conversion from MCI to Alzheimer's disease. In particular, the latent space of a variational auto-encoder network trained with the MCI and Alzheimer's patients is manipulated to obtain the significant attributes and decipher their behavior that leads to the conversion from MCI to Alzheimer's disease. By utilizing a generative decoder and the dimensions that lead to the Alzheimer's diagnosis, we generate synthetic dementia patients from MCI patients in the dataset. Experimental results show promising quantitative and qualitative results on one of the most extensive and commonly used Alzheimer's disease neuroimaging datasets in literature.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge