Claudio L. A. Bassetti
Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
NAP: Attention-Based Late Fusion for Automatic Sleep Staging
Nov 05, 2025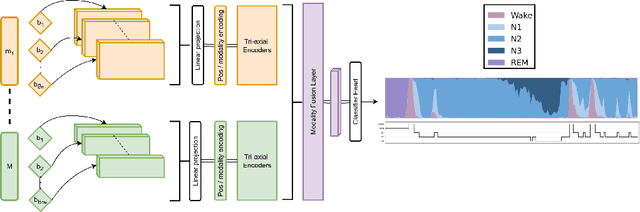
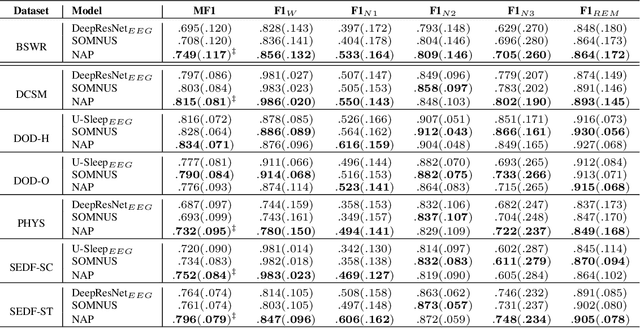
Abstract:Polysomnography signals are highly heterogeneous, varying in modality composition (e.g., EEG, EOG, ECG), channel availability (e.g., frontal, occipital EEG), and acquisition protocols across datasets and clinical sites. Most existing models that process polysomnography data rely on a fixed subset of modalities or channels and therefore neglect to fully exploit its inherently multimodal nature. We address this limitation by introducing NAP (Neural Aggregator of Predictions), an attention-based model which learns to combine multiple prediction streams using a tri-axial attention mechanism that captures temporal, spatial, and predictor-level dependencies. NAP is trained to adapt to different input dimensions. By aggregating outputs from frozen, pretrained single-channel models, NAP consistently outperforms individual predictors and simple ensembles, achieving state-of-the-art zero-shot generalization across multiple datasets. While demonstrated in the context of automated sleep staging from polysomnography, the proposed approach could be extended to other multimodal physiological applications.
SLEEPYLAND: trust begins with fair evaluation of automatic sleep staging models
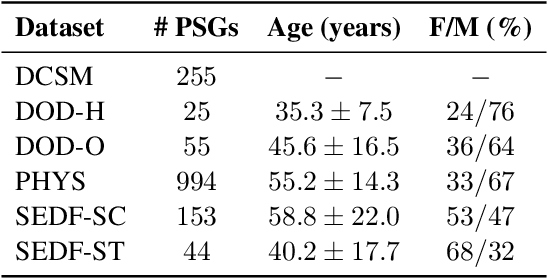
Jun 11, 2025Abstract:Despite advances in deep learning for automatic sleep staging, clinical adoption remains limited due to challenges in fair model evaluation, generalization across diverse datasets, model bias, and variability in human annotations. We present SLEEPYLAND, an open-source sleep staging evaluation framework designed to address these barriers. It includes more than 220'000 hours in-domain (ID) sleep recordings, and more than 84'000 hours out-of-domain (OOD) sleep recordings, spanning a broad range of ages, sleep-wake disorders, and hardware setups. We release pre-trained models based on high-performing SoA architectures and evaluate them under standardized conditions across single- and multi-channel EEG/EOG configurations. We introduce SOMNUS, an ensemble combining models across architectures and channel setups via soft voting. SOMNUS achieves robust performance across twenty-four different datasets, with macro-F1 scores between 68.7% and 87.2%, outperforming individual models in 94.9% of cases. Notably, SOMNUS surpasses previous SoA methods, even including cases where compared models were trained ID while SOMNUS treated the same data as OOD. Using a subset of the BSWR (N=6'633), we quantify model biases linked to age, gender, AHI, and PLMI, showing that while ensemble improves robustness, no model architecture consistently minimizes bias in performance and clinical markers estimation. In evaluations on OOD multi-annotated datasets (DOD-H, DOD-O), SOMNUS exceeds the best human scorer, i.e., MF1 85.2% vs 80.8% on DOD-H, and 80.2% vs 75.9% on DOD-O, better reproducing the scorer consensus than any individual expert (k = 0.89/0.85 and ACS = 0.95/0.94 for healthy/OSA cohorts). Finally, we introduce ensemble disagreement metrics - entropy and inter-model divergence based - predicting regions of scorer disagreement with ROC AUCs up to 0.828, offering a data-driven proxy for human uncertainty.
Bridging AI and Clinical Practice: Integrating Automated Sleep Scoring Algorithm with Uncertainty-Guided Physician Review
Dec 22, 2023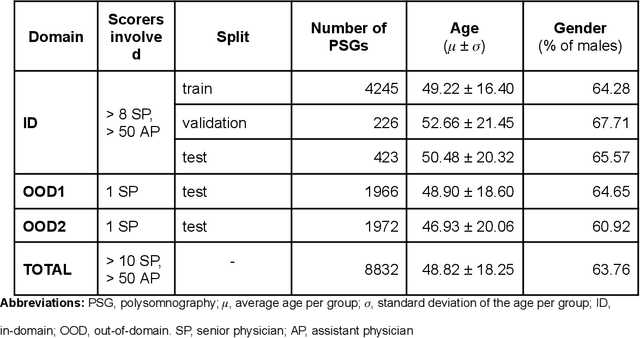
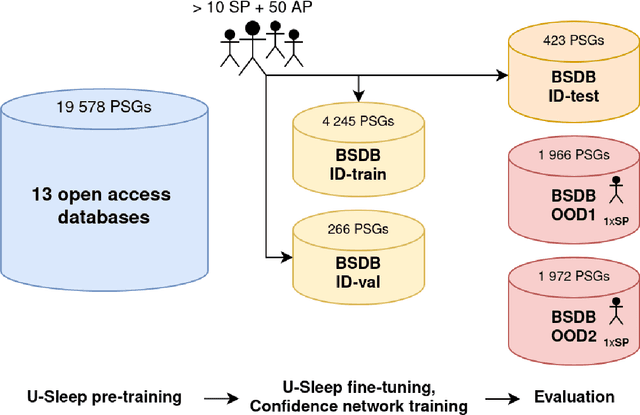

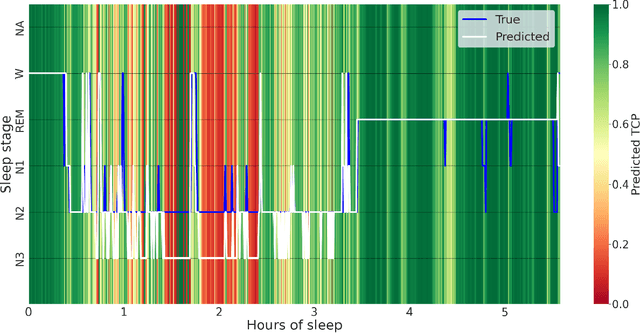
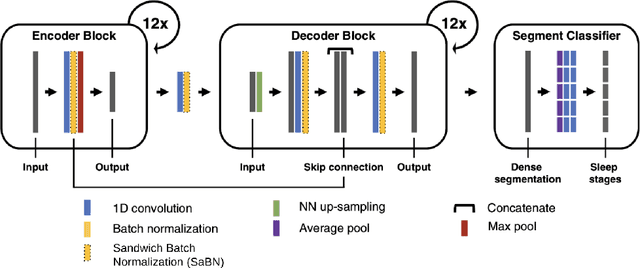
Abstract:Purpose: This study aims to enhance the clinical use of automated sleep-scoring algorithms by incorporating an uncertainty estimation approach to efficiently assist clinicians in the manual review of predicted hypnograms, a necessity due to the notable inter-scorer variability inherent in polysomnography (PSG) databases. Our efforts target the extent of review required to achieve predefined agreement levels, examining both in-domain and out-of-domain data, and considering subjects diagnoses. Patients and methods: Total of 19578 PSGs from 13 open-access databases were used to train U-Sleep, a state-of-the-art sleep-scoring algorithm. We leveraged a comprehensive clinical database of additional 8832 PSGs, covering a full spectrum of ages and sleep-disorders, to refine the U-Sleep, and to evaluate different uncertainty-quantification approaches, including our novel confidence network. The ID data consisted of PSGs scored by over 50 physicians, and the two OOD sets comprised recordings each scored by a unique senior physician. Results: U-Sleep demonstrated robust performance, with Cohen's kappa (K) at 76.2% on ID and 73.8-78.8% on OOD data. The confidence network excelled at identifying uncertain predictions, achieving AUROC scores of 85.7% on ID and 82.5-85.6% on OOD data. Independently of sleep-disorder status, statistical evaluations revealed significant differences in confidence scores between aligning vs discording predictions, and significant correlations of confidence scores with classification performance metrics. To achieve K of at least 90% with physician intervention, examining less than 29.0% of uncertain epochs was required, substantially reducing physicians workload, and facilitating near-perfect agreement.
U-Sleep: resilient to AASM guidelines
Sep 23, 2022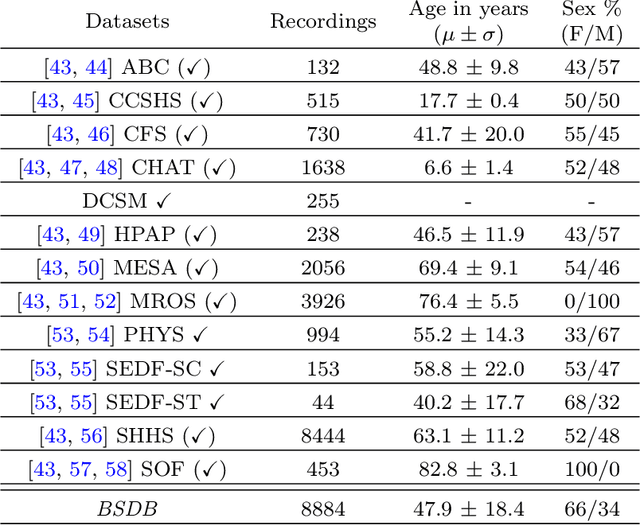
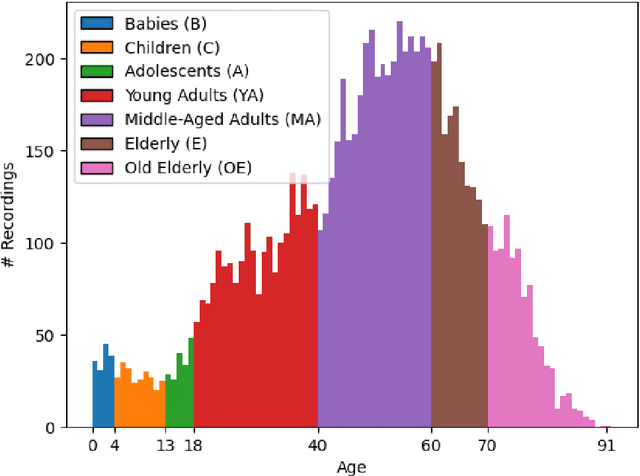
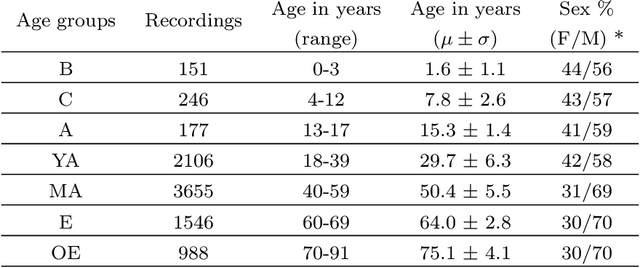
Abstract:AASM guidelines are the results of decades of efforts aiming at standardizing sleep scoring procedure, in order to have a commonly used methodology. The guidelines cover several aspects from the technical/digital specifications, e.g., recommended EEG derivations, to detailed sleep scoring rules accordingly to age. In the context of sleep scoring automation, deep learning has demonstrated better performance compared to many other techniques. Usually, clinical expertise and official guidelines are fundamental to support automated sleep scoring algorithms in solving the task. In this paper we show that a deep learning based sleep scoring algorithm may not need to fully exploit the clinical knowledge or to strictly follow the AASM guidelines. Specifically, we demonstrate that U-Sleep, a state-of-the-art sleep scoring algorithm, can be strong enough to solve the scoring task even using clinically non-recommended or non-conventional derivations, and with no need to exploit information about the chronological age of the subjects. We finally strengthen a well-known finding that using data from multiple data centers always results in a better performing model compared with training on a single cohort. Indeed, we show that this latter statement is still valid even by increasing the size and the heterogeneity of the single data cohort. In all our experiments we used 28528 polysomnography studies from 13 different clinical studies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge