Chun-Ren Phang
Multiagent Copilot Approach for Shared Autonomy between Human EEG and TD3 Deep Reinforcement Learning
Dec 22, 2023Abstract:Deep reinforcement learning (RL) algorithms enable the development of fully autonomous agents that can interact with the environment. Brain-computer interface (BCI) systems decipher human implicit brain signals regardless of the explicit environment. In this study, we integrated deep RL and BCI to improve beneficial human interventions in autonomous systems and the performance in decoding brain activities by considering environmental factors. Shared autonomy was allowed between the action command decoded from the electroencephalography (EEG) of the human agent and the action generated from the twin delayed DDPG (TD3) agent for a given environment. Our proposed copilot control scheme with a full blocker (Co-FB) significantly outperformed the individual EEG (EEG-NB) or TD3 control. The Co-FB model achieved a higher target approaching score, lower failure rate, and lower human workload than the EEG-NB model. The Co-FB control scheme had a higher invisible target score and level of allowed human intervention than the TD3 model. We also proposed a disparity d-index to evaluate the effect of contradicting agent decisions on the control accuracy and authority of the copilot model. We found a significant correlation between the control authority of the TD3 agent and the performance improvement of human EEG classification with respect to the d-index. We also observed that shifting control authority to the TD3 agent improved performance when BCI decoding was not optimal. These findings indicate that the copilot system can effectively handle complex environments and that BCI performance can be improved by considering environmental factors. Future work should employ continuous action space and different multi-agent approaches to evaluate copilot performance.
Classification of EEG-Based Brain Connectivity Networks in Schizophrenia Using a Multi-Domain Connectome Convolutional Neural Network
Mar 21, 2019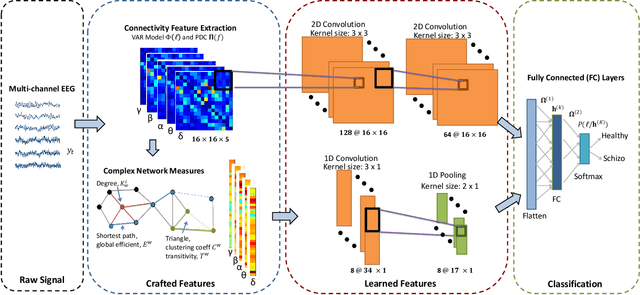
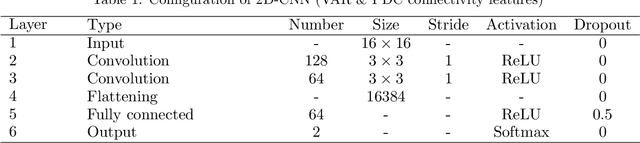
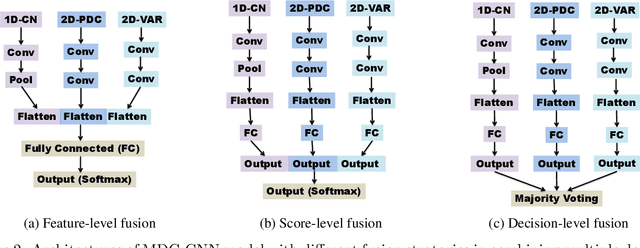

Abstract:We exploit altered patterns in brain functional connectivity as features for automatic discriminative analysis of neuropsychiatric patients. Deep learning methods have been introduced to functional network classification only very recently for fMRI, and the proposed architectures essentially focused on a single type of connectivity measure. We propose a deep convolutional neural network (CNN) framework for classification of electroencephalogram (EEG)-derived brain connectome in schizophrenia (SZ). To capture complementary aspects of disrupted connectivity in SZ, we explore combination of various connectivity features consisting of time and frequency-domain metrics of effective connectivity based on vector autoregressive model and partial directed coherence, and complex network measures of network topology. We design a novel multi-domain connectome CNN (MDC-CNN) based on a parallel ensemble of 1D and 2D CNNs to integrate the features from various domains and dimensions using different fusion strategies. Hierarchical latent representations learned by the multiple convolutional layers from EEG connectivity reveal apparent group differences between SZ and healthy controls (HC). Results on a large resting-state EEG dataset show that the proposed CNNs significantly outperform traditional support vector machine classifiers. The MDC-CNN with combined connectivity features further improves performance over single-domain CNNs using individual features, achieving remarkable accuracy of $93.06\%$ with a decision-level fusion. The proposed MDC-CNN by integrating information from diverse brain connectivity descriptors is able to accurately discriminate SZ from HC. The new framework is potentially useful for developing diagnostic tools for SZ and other disorders.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge