Carla Floricel
DASS Good: Explainable Data Mining of Spatial Cohort Data
Apr 10, 2023Abstract:Developing applicable clinical machine learning models is a difficult task when the data includes spatial information, for example, radiation dose distributions across adjacent organs at risk. We describe the co-design of a modeling system, DASS, to support the hybrid human-machine development and validation of predictive models for estimating long-term toxicities related to radiotherapy doses in head and neck cancer patients. Developed in collaboration with domain experts in oncology and data mining, DASS incorporates human-in-the-loop visual steering, spatial data, and explainable AI to augment domain knowledge with automatic data mining. We demonstrate DASS with the development of two practical clinical stratification models and report feedback from domain experts. Finally, we describe the design lessons learned from this collaborative experience.
Visual Analysis and Detection of Contrails in Aircraft Engine Simulations
Aug 08, 2022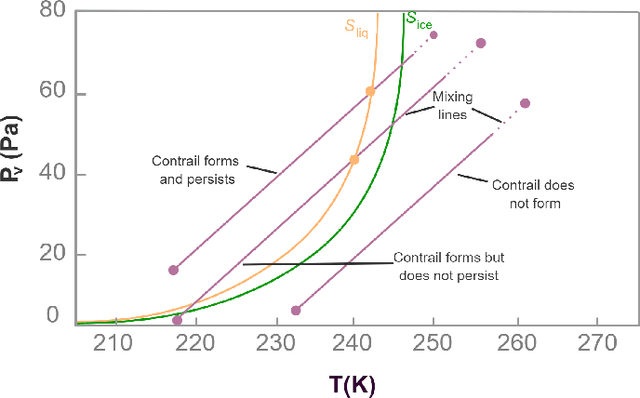
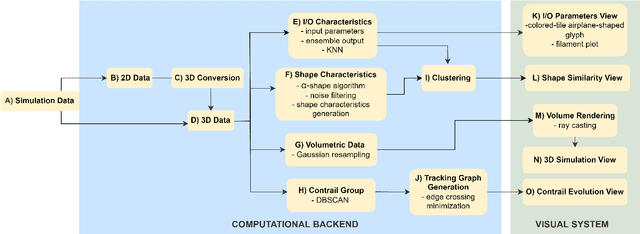
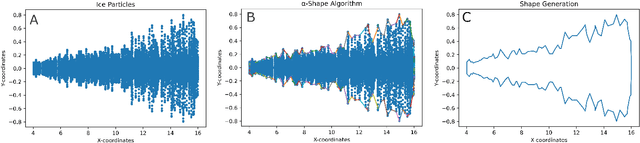
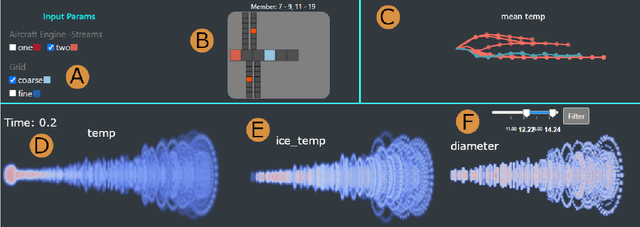
Abstract:Contrails are condensation trails generated from emitted particles by aircraft engines, which perturb Earth's radiation budget. Simulation modeling is used to interpret the formation and development of contrails. These simulations are computationally intensive and rely on high-performance computing solutions, and the contrail structures are not well defined. We propose a visual computing system to assist in defining contrails and their characteristics, as well as in the analysis of parameters for computer-generated aircraft engine simulations. The back-end of our system leverages a contrail-formation criterion and clustering methods to detect contrails' shape and evolution and identify similar simulation runs. The front-end system helps analyze contrails and their parameters across multiple simulation runs. The evaluation with domain experts shows this approach successfully aids in contrail data investigation.
THALIS: Human-Machine Analysis of Longitudinal Symptoms in Cancer Therapy
Aug 05, 2021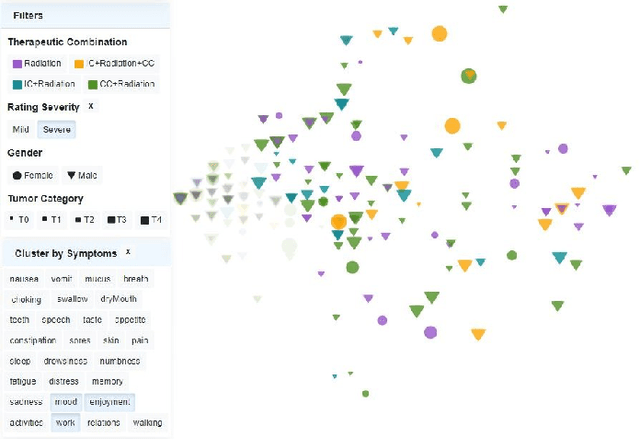
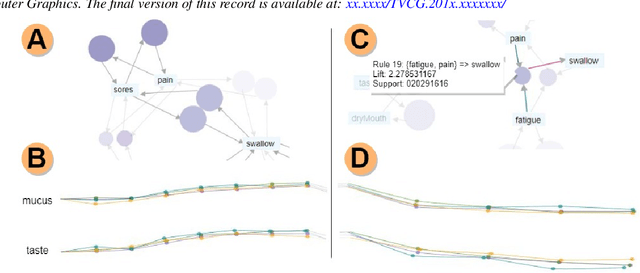
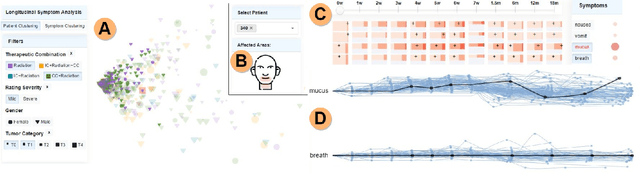
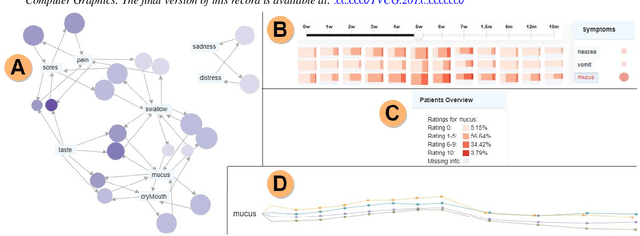
Abstract:Although cancer patients survive years after oncologic therapy, they are plagued with long-lasting or permanent residual symptoms, whose severity, rate of development, and resolution after treatment vary largely between survivors. The analysis and interpretation of symptoms is complicated by their partial co-occurrence, variability across populations and across time, and, in the case of cancers that use radiotherapy, by further symptom dependency on the tumor location and prescribed treatment. We describe THALIS, an environment for visual analysis and knowledge discovery from cancer therapy symptom data, developed in close collaboration with oncology experts. Our approach leverages unsupervised machine learning methodology over cohorts of patients, and, in conjunction with custom visual encodings and interactions, provides context for new patients based on patients with similar diagnostic features and symptom evolution. We evaluate this approach on data collected from a cohort of head and neck cancer patients. Feedback from our clinician collaborators indicates that THALIS supports knowledge discovery beyond the limits of machines or humans alone, and that it serves as a valuable tool in both the clinic and symptom research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge