Benedek Harsanyi
Towards generalizable single-cell perturbation modeling via the Conditional Monge Gap
Apr 11, 2025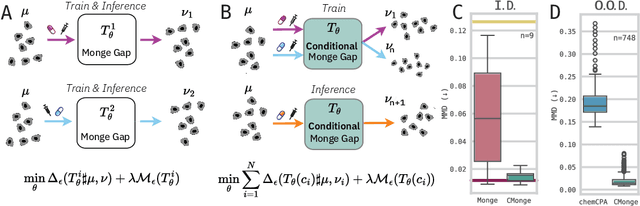
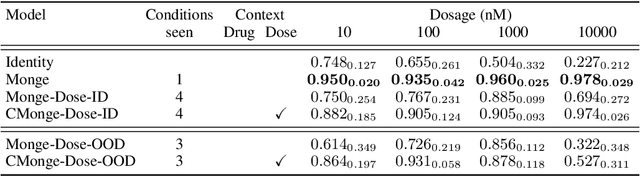
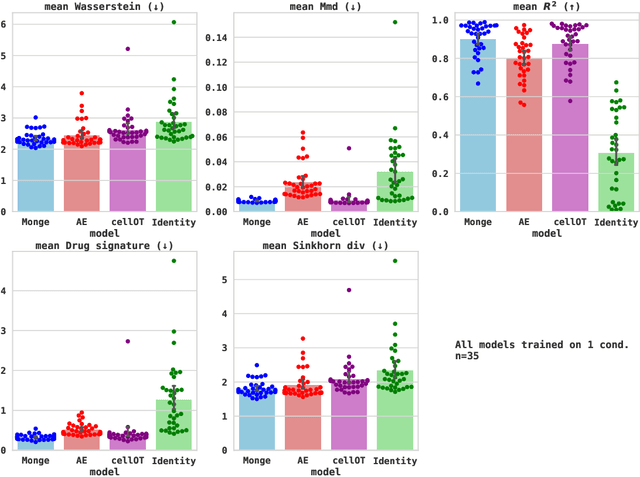

Abstract:Learning the response of single-cells to various treatments offers great potential to enable targeted therapies. In this context, neural optimal transport (OT) has emerged as a principled methodological framework because it inherently accommodates the challenges of unpaired data induced by cell destruction during data acquisition. However, most existing OT approaches are incapable of conditioning on different treatment contexts (e.g., time, drug treatment, drug dosage, or cell type) and we still lack methods that unanimously show promising generalization performance to unseen treatments. Here, we propose the Conditional Monge Gap which learns OT maps conditionally on arbitrary covariates. We demonstrate its value in predicting single-cell perturbation responses conditional to one or multiple drugs, a drug dosage, or combinations thereof. We find that our conditional models achieve results comparable and sometimes even superior to the condition-specific state-of-the-art on scRNA-seq as well as multiplexed protein imaging data. Notably, by aggregating data across conditions we perform cross-task learning which unlocks remarkable generalization abilities to unseen drugs or drug dosages, widely outperforming other conditional models in capturing heterogeneity (i.e., higher moments) in the perturbed population. Finally, by scaling to hundreds of conditions and testing on unseen drugs, we narrow the gap between structure-based and effect-based drug representations, suggesting a promising path to the successful prediction of perturbation effects for unseen treatments.
Quantum Theory and Application of Contextual Optimal Transport
Feb 22, 2024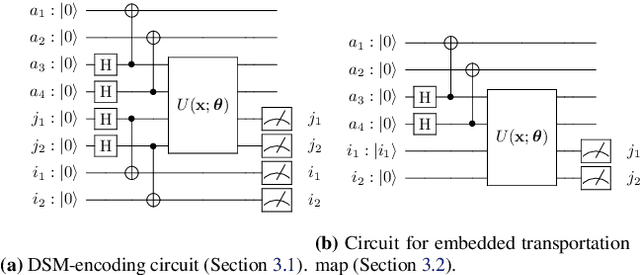
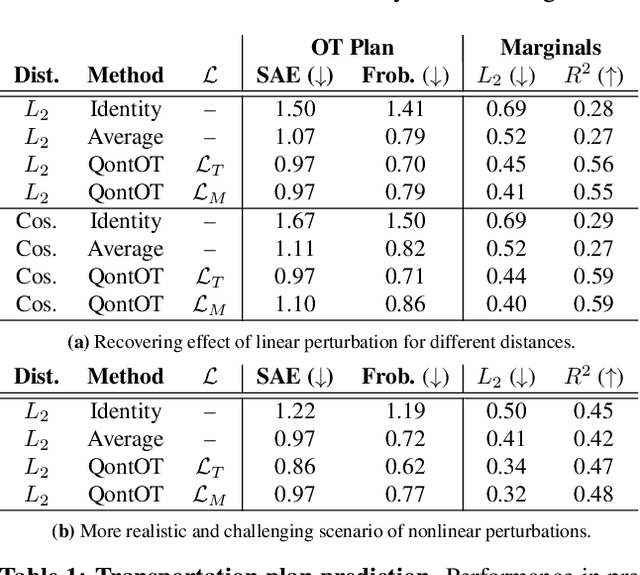
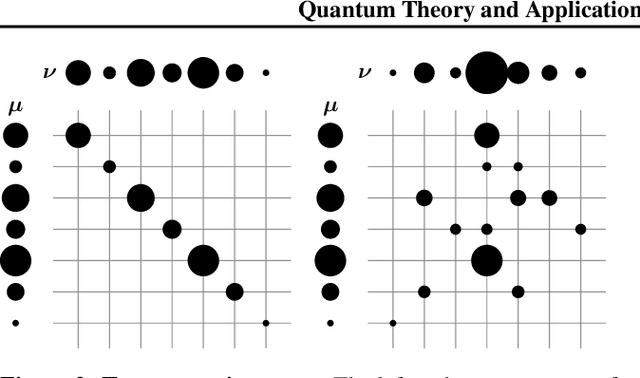
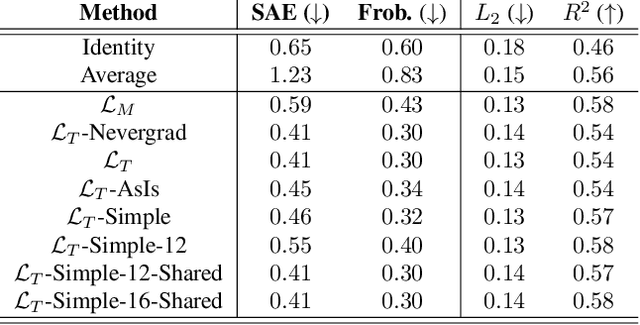
Abstract:Optimal Transport (OT) has fueled machine learning (ML) applications across many domains. In cases where paired data measurements ($\mu$, $\nu$) are coupled to a context variable $p_i$ , one may aspire to learn a global transportation map that can be parameterized through a potentially unseen con-text. Existing approaches utilize Neural OT and largely rely on Brenier's theorem. Here, we propose a first-of-its-kind quantum computing formulation for amortized optimization of contextualized transportation plans. We exploit a direct link between doubly stochastic matrices and unitary operators thus finding a natural connection between OT and quantum computation. We verify our method on synthetic and real data, by predicting variations in cell type distributions parameterized through drug dosage as context. Our comparisons to several baselines reveal that our method can capture dose-induced variations in cell distributions, even to some extent when dosages are extrapolated and sometimes with performance similar to the best classical models. In summary, this is a first step toward learning to predict contextualized transportation plans through quantum.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge