Axel Camara
Self-Supervision Closes the Gap Between Weak and Strong Supervision in Histology
Dec 07, 2020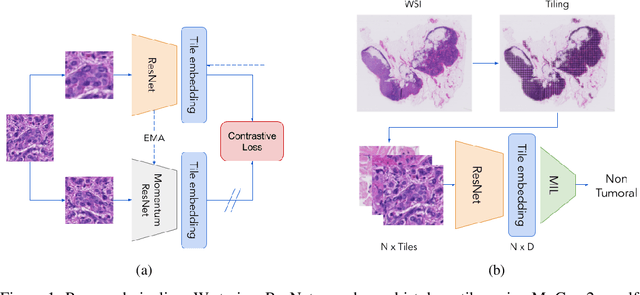
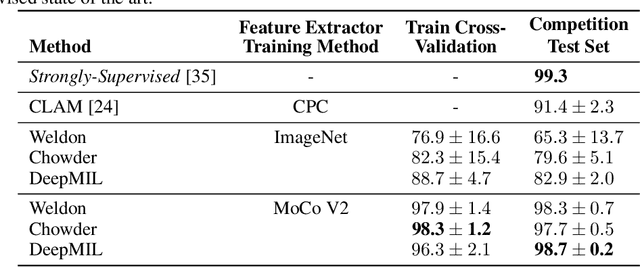
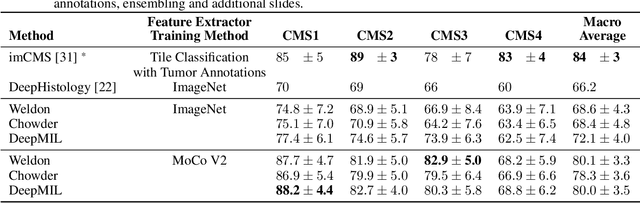
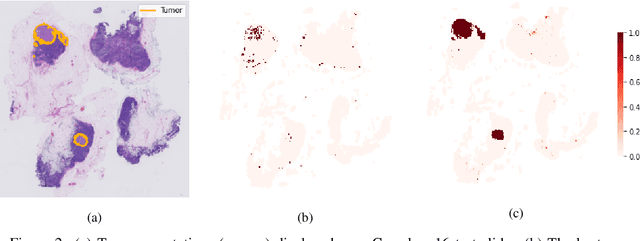
Abstract:One of the biggest challenges for applying machine learning to histopathology is weak supervision: whole-slide images have billions of pixels yet often only one global label. The state of the art therefore relies on strongly-supervised model training using additional local annotations from domain experts. However, in the absence of detailed annotations, most weakly-supervised approaches depend on a frozen feature extractor pre-trained on ImageNet. We identify this as a key weakness and propose to train an in-domain feature extractor on histology images using MoCo v2, a recent self-supervised learning algorithm. Experimental results on Camelyon16 and TCGA show that the proposed extractor greatly outperforms its ImageNet counterpart. In particular, our results improve the weakly-supervised state of the art on Camelyon16 from 91.4% to 98.7% AUC, thereby closing the gap with strongly-supervised models that reach 99.3% AUC. Through these experiments, we demonstrate that feature extractors trained via self-supervised learning can act as drop-in replacements to significantly improve existing machine learning techniques in histology. Lastly, we show that the learned embedding space exhibits biologically meaningful separation of tissue structures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge