Asif Iftekhar Omi
A Systematic Method for Optimum Biomedical Wireless Power Transfer using Inductive Links in Area-Constrained Implants
Jan 15, 2025



Abstract:In the context of implantable bioelectronics, this work provides new insights into maximizing biomedical wireless power transfer (BWPT) via the systematic development of inductive links. This approach addresses the specific challenges of power transfer efficiency (PTE) optimization within the area constraints of bio-implants embedded in tissue. Key contributions include the derivation of an optimal self-inductance with S-parameter-based analyses leading to the co-design of planar spiral coils and L-section impedance matching networks. To validate the proposed design methodology, two coil prototypes -- one symmetric (type-1) and one asymmetric (type-2) -- were fabricated and tested for PTE in pork tissue. Targeting a 20 MHz design frequency, the type-1 coil demonstrated a state-of-the-art PTE of $\sim$ 4\% (channel length = 15 mm) with a return loss (RL) $>$ 20 dB on both the input and output sides, within an area constraint of $<$ 18 $ \times $ 18 mm$^{2}$. In contrast, the type-2 coil achieved a PTE of $\sim$ 2\% with an RL $>$ 15 dB, for a smaller receiving coil area of $<$ 5x5 mm$^{2}$ for the same tissue environment. To complement the coils, we demonstrate a 65 nm test chip with an integrated energy harvester, which includes \asif{a} 30-stage rectifier and low-dropout regulator (LDO), producing a stable $\sim$ 1V DC output within tissue medium, matching theoretical predictions and simulations. Furthermore, we provide a robust and comprehensive guideline for advancing efficient inductive links for various BWPT applications, with shared resources in GitHub available for utilization by the broader community.
Galvanic Body-Coupled Powering for Wireless Implanted Neurostimulators
Dec 04, 2024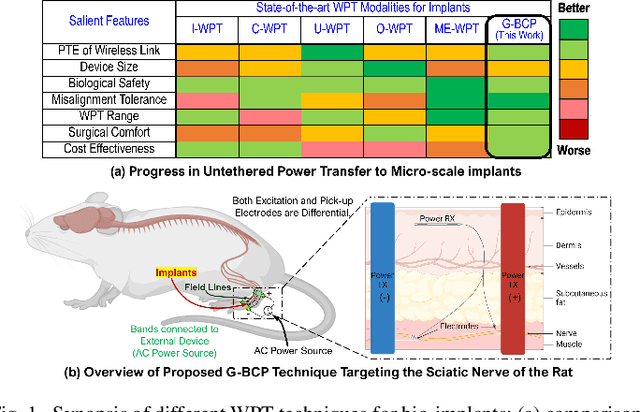
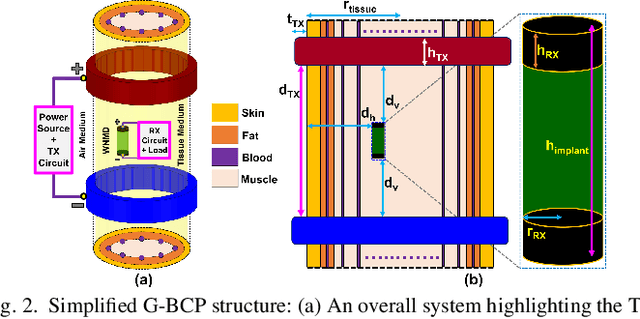
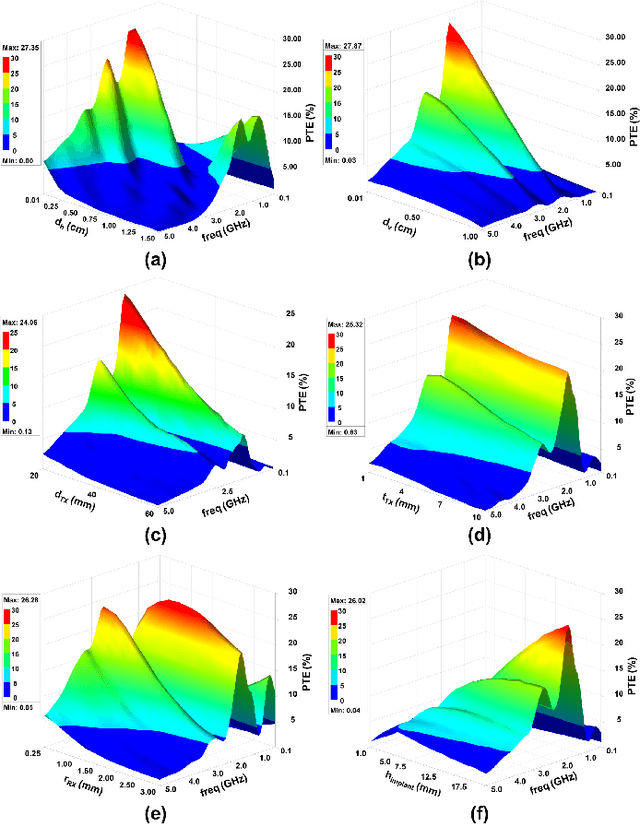
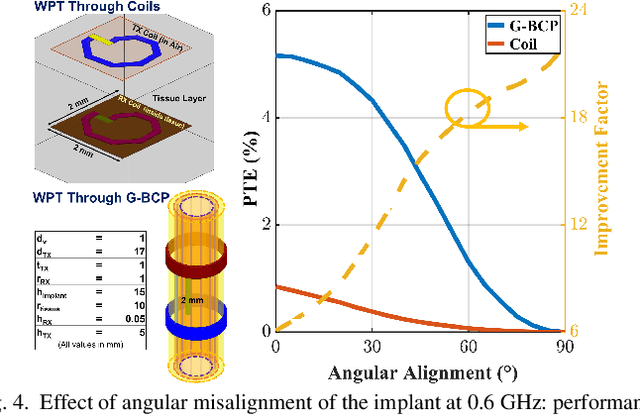
Abstract:Body-coupled powering (BCP) is an innovative wireless power transfer (WPT) technique, recently explored for its potential to deliver power to cutting-edge biomedical implants such as nerve and muscle stimulators. This paper demonstrates the efficient technique of designing WPT systems embedding BCP via galvanic coupling (G-BCP). The G-BCP configuration utilizes two metal circular rings surrounding the body area of interest as the transmitter (TX) electrodes required for galvanic (differential) excitation and a wireless implant as the receiver (RX) equipped with two electrodes for differential power reception accordingly. By focusing on the unique advantages of this approach - such as enhanced targeting accuracy, improved power transfer efficiency (PTE), and favorable tissue penetration characteristics, G-BCP emerges as a superior alternative to traditional WPT methods. A comprehensive analysis is conducted to obtain the optimized device parameters while simultaneously allowing flexible placement of implants at different depths and alignments. To substantiate the proposed design concept, a prototype was simulated in Ansys HFSS, employing a multi-layered tissue medium of 10mm radius and targeting the sciatic nerve of a rat. Impressively, this prototype achieves > 20% PTE at 1.25 GHz, with the implant (radius of RX electrodes = 1 mm) located 2 mm deep inside the tissue model having complex load impedance of Rload = 1000 Ohm and Cload = 5pF. Therefore, the G-BCP-based wirelessly powered microdevices are envisaged to be a key enabler in neural recording and stimulation, specifically for the peripheral nervous system, enhancing therapeutic outcomes and patient experiences.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge