Arezoo Borji
A Review on the Integration of Artificial Intelligence and Medical Imaging in IVF Ovarian Stimulation
Dec 27, 2024



Abstract:Artificial intelligence (AI) has emerged as a powerful tool to enhance decision-making and optimize treatment protocols in in vitro fertilization (IVF). In particular, AI shows significant promise in supporting decision-making during the ovarian stimulation phase of the IVF process. This review evaluates studies focused on the applications of AI combined with medical imaging in ovarian stimulation, examining methodologies, outcomes, and current limitations. Our analysis of 13 studies on this topic reveals that, reveal that while AI algorithms demonstrated notable potential in predicting optimal hormonal dosages, trigger timing, and oocyte retrieval outcomes, the medical imaging data utilized predominantly came from two-dimensional (2D) ultrasound which mainly involved basic quantifications, such as follicle size and number, with limited use of direct feature extraction or advanced image analysis techniques. This points to an underexplored opportunity where advanced image analysis approaches, such as deep learning, and more diverse imaging modalities, like three-dimensional (3D) ultrasound, could unlock deeper insights. Additionally, the lack of explainable AI (XAI) in most studies raises concerns about the transparency and traceability of AI-driven decisions - key factors for clinical adoption and trust. Furthermore, many studies relied on single-center designs and small datasets, which limit the generalizability of their findings. This review highlights the need for integrating advanced imaging analysis techniques with explainable AI methodologies, as well as the importance of leveraging multicenter collaborations and larger datasets. Addressing these gaps has the potential to enhance ovarian stimulation management, paving the way for efficient, personalized, and data-driven treatment pathways that improve IVF outcomes.
An Integrated Optimization and Deep Learning Pipeline for Predicting Live Birth Success in IVF Using Feature Optimization and Transformer-Based Models
Dec 27, 2024Abstract:In vitro fertilization (IVF) is a widely utilized assisted reproductive technology, yet predicting its success remains challenging due to the multifaceted interplay of clinical, demographic, and procedural factors. This study develops a robust artificial intelligence (AI) pipeline aimed at predicting live birth outcomes in IVF treatments. The pipeline uses anonymized data from 2010 to 2018, obtained from the Human Fertilization and Embryology Authority (HFEA). We evaluated the prediction performance of live birth success as a binary outcome (success/failure) by integrating different feature selection methods, such as principal component analysis (PCA) and particle swarm optimization (PSO), with different traditional machine learning-based classifiers including random forest (RF) and decision tree, as well as deep learning-based classifiers including custom transformer-based model and a tab transformer model with an attention mechanism. Our research demonstrated that the best performance was achieved by combining PSO for feature selection with the TabTransformer-based deep learning model, yielding an accuracy of 99.50% and an AUC of 99.96%, highlighting its significant performance to predict live births. This study establishes a highly accurate AI pipeline for predicting live birth outcomes in IVF, demonstrating its potential to enhance personalized fertility treatments.
Advanced Hybrid Deep Learning Model for Enhanced Classification of Osteosarcoma Histopathology Images
Oct 29, 2024



Abstract:Recent advances in machine learning are transforming medical image analysis, particularly in cancer detection and classification. Techniques such as deep learning, especially convolutional neural networks (CNNs) and vision transformers (ViTs), are now enabling the precise analysis of complex histopathological images, automating detection, and enhancing classification accuracy across various cancer types. This study focuses on osteosarcoma (OS), the most common bone cancer in children and adolescents, which affects the long bones of the arms and legs. Early and accurate detection of OS is essential for improving patient outcomes and reducing mortality. However, the increasing prevalence of cancer and the demand for personalized treatments create challenges in achieving precise diagnoses and customized therapies. We propose a novel hybrid model that combines convolutional neural networks (CNN) and vision transformers (ViT) to improve diagnostic accuracy for OS using hematoxylin and eosin (H&E) stained histopathological images. The CNN model extracts local features, while the ViT captures global patterns from histopathological images. These features are combined and classified using a Multi-Layer Perceptron (MLP) into four categories: non-tumor (NT), non-viable tumor (NVT), viable tumor (VT), and none-viable ratio (NVR). Using the Cancer Imaging Archive (TCIA) dataset, the model achieved an accuracy of 99.08%, precision of 99.10%, recall of 99.28%, and an F1-score of 99.23%. This is the first successful four-class classification using this dataset, setting a new benchmark in OS research and offering promising potential for future diagnostic advancements.
Introducing an ensemble method for the early detection of Alzheimer's disease through the analysis of PET scan images
Apr 01, 2024Abstract:Alzheimer's disease is a progressive neurodegenerative disorder that primarily affects cognitive functions such as memory, thinking, and behavior. In this disease, there is a critical phase, mild cognitive impairment, that is really important to be diagnosed early since some patients with progressive MCI will develop the disease. This study delves into the challenging task of classifying Alzheimer's disease into four distinct groups: control normal (CN), progressive mild cognitive impairment (pMCI), stable mild cognitive impairment (sMCI), and Alzheimer's disease (AD). This classification is based on a thorough examination of PET scan images obtained from the ADNI dataset, which provides a thorough understanding of the disease's progression. Several deep-learning and traditional machine-learning models have been used to detect Alzheimer's disease. In this paper, three deep-learning models, namely VGG16 and AlexNet, and a custom Convolutional neural network (CNN) with 8-fold cross-validation have been used for classification. Finally, an ensemble technique is used to improve the overall result of these models. The results show that using deep-learning models to tell the difference between MCI patients gives an overall average accuracy of 93.13% and an AUC of 94.4%.
A learning-based solution approach to the application placement problem in mobile edge computing under uncertainty
Mar 23, 2024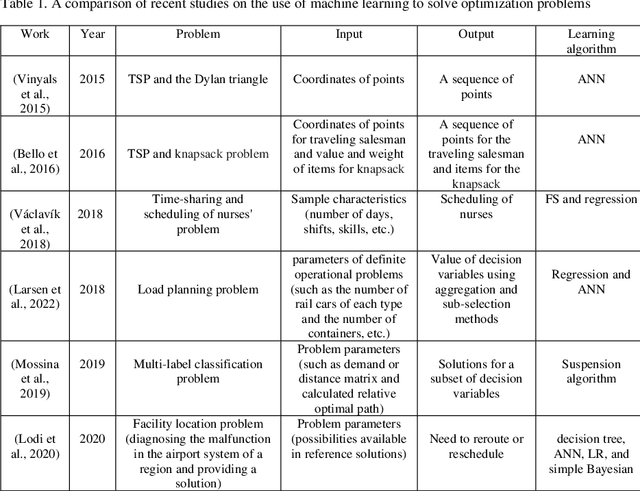

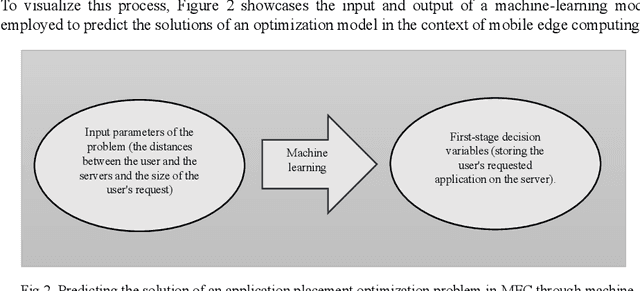
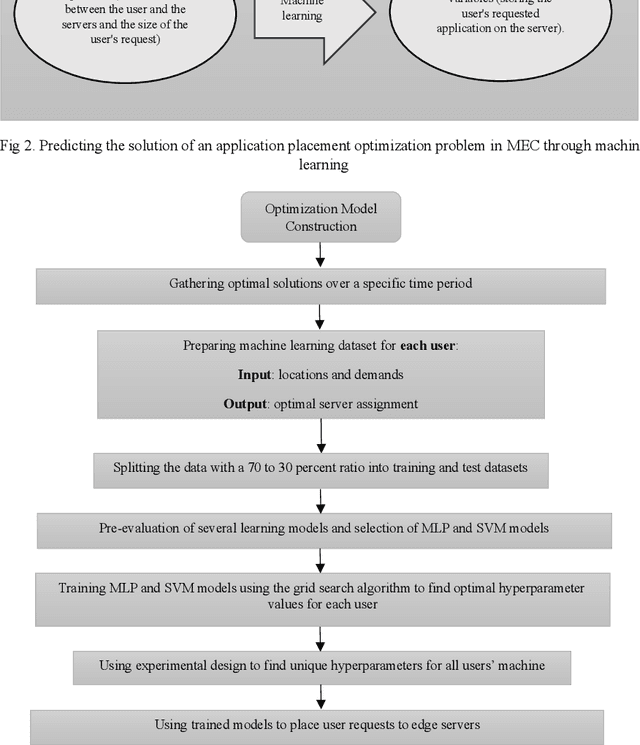
Abstract:Placing applications in mobile edge computing servers presents a complex challenge involving many servers, users, and their requests. Existing algorithms take a long time to solve high-dimensional problems with significant uncertainty scenarios. Therefore, an efficient approach is required to maximize the quality of service while considering all technical constraints. One of these approaches is machine learning, which emulates optimal solutions for application placement in edge servers. Machine learning models are expected to learn how to allocate user requests to servers based on the spatial positions of users and servers. In this study, the problem is formulated as a two-stage stochastic programming. A sufficient amount of training records is generated by varying parameters such as user locations, their request rates, and solving the optimization model. Then, based on the distance features of each user from the available servers and their request rates, machine learning models generate decision variables for the first stage of the stochastic optimization model, which is the user-to-server request allocation, and are employed as independent decision agents that reliably mimic the optimization model. Support Vector Machines (SVM) and Multi-layer Perceptron (MLP) are used in this research to achieve practical decisions from the stochastic optimization models. The performance of each model has shown an execution effectiveness of over 80%. This research aims to provide a more efficient approach for tackling high-dimensional problems and scenarios with uncertainties in mobile edge computing by leveraging machine learning models for optimal decision-making in request allocation to edge servers. These results suggest that machine-learning models can significantly improve solution times compared to conventional approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge