Andriy Temko
Neonatal seizure detection from raw multi-channel EEG using a fully convolutional architecture
May 28, 2021



Abstract:A deep learning classifier for detecting seizures in neonates is proposed. This architecture is designed to detect seizure events from raw electroencephalogram (EEG) signals as opposed to the state-of-the-art hand engineered feature-based representation employed in traditional machine learning based solutions. The seizure detection system utilises only convolutional layers in order to process the multichannel time domain signal and is designed to exploit the large amount of weakly labelled data in the training stage. The system performance is assessed on a large database of continuous EEG recordings of 834h in duration; this is further validated on a held-out publicly available dataset and compared with two baseline SVM based systems. The developed system achieves a 56% relative improvement with respect to a feature-based state-of-the art baseline, reaching an AUC of 98.5%; this also compares favourably both in terms of performance and run-time. The effect of varying architectural parameters is thoroughly studied. The performance improvement is achieved through novel architecture design which allows more efficient usage of available training data and end-to-end optimisation from the front-end feature extraction to the back-end classification. The proposed architecture opens new avenues for the application of deep learning to neonatal EEG, where the performance becomes a function of the amount of training data with less dependency on the availability of precise clinical labels.
Deep Learning for EEG Seizure Detection in Preterm Infants
May 28, 2021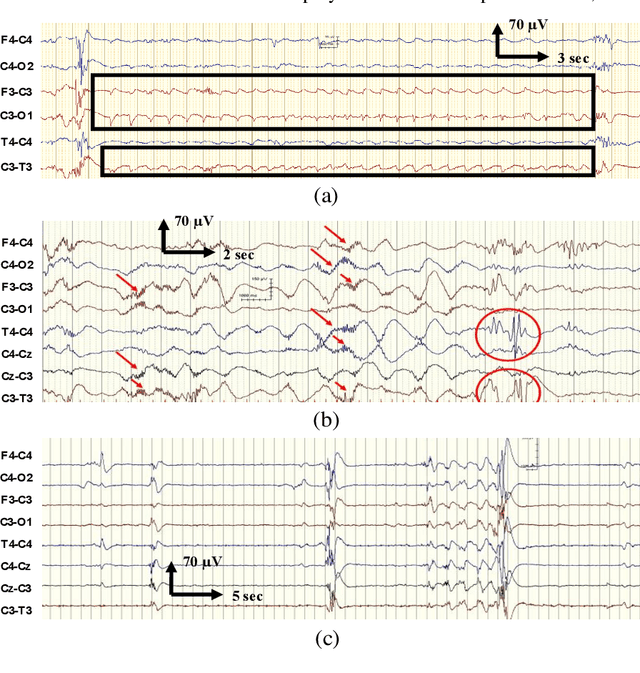
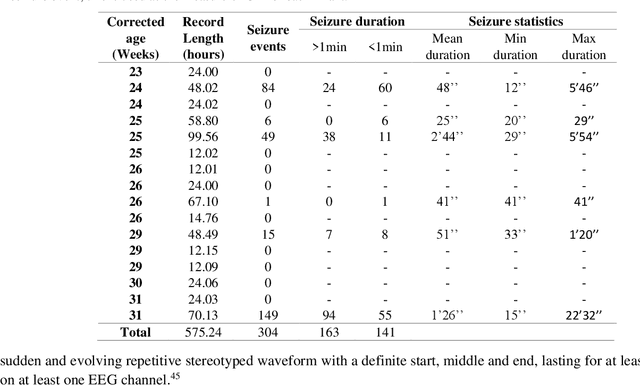
Abstract:EEG is the gold standard for seizure detection in the newborn infant, but EEG interpretation in the preterm group is particularly challenging; trained experts are scarce and the task of interpreting EEG in real-time is arduous. Preterm infants are reported to have a higher incidence of seizures compared to term infants. Preterm EEG morphology differs from that of term infants, which implies that seizure detection algorithms trained on term EEG may not be appropriate. The task of developing preterm specific algorithms becomes extra-challenging given the limited amount of annotated preterm EEG data available. This paper explores novel deep learning (DL) architectures for the task of neonatal seizure detection in preterm infants. The study tests and compares several approaches to address the problem: training on data from full-term infants; training on data from preterm infants; training on age-specific preterm data and transfer learning. The system performance is assessed on a large database of continuous EEG recordings of 575h in duration. It is shown that the accuracy of a validated term-trained EEG seizure detection algorithm, based on a support vector machine classifier, when tested on preterm infants falls well short of the performance achieved for full-term infants. An AUC of 88.3% was obtained when tested on preterm EEG as compared to 96.6% obtained when tested on term EEG. When re-trained on preterm EEG, the performance marginally increases to 89.7%. An alternative DL approach shows a more stable trend when tested on the preterm cohort, starting with an AUC of 93.3% for the term-trained algorithm and reaching 95.0% by transfer learning from the term model using available preterm data.
Neonatal EEG Interpretation and Decision Support Framework for Mobile Platforms
Jun 08, 2018



Abstract:This paper proposes and implements an intuitive and pervasive solution for neonatal EEG monitoring assisted by sonification and deep learning AI that provides information about neonatal brain health to all neonatal healthcare professionals, particularly those without EEG interpretation expertise. The system aims to increase the demographic of clinicians capable of diagnosing abnormalities in neonatal EEG. The proposed system uses a low-cost and low-power EEG acquisition system. An Android app provides single-channel EEG visualization, traffic-light indication of the presence of neonatal seizures provided by a trained, deep convolutional neural network and an algorithm for EEG sonification, designed to facilitate the perception of changes in EEG morphology specific to neonatal seizures. The multifaceted EEG interpretation framework is presented and the implemented mobile platform architecture is analyzed with respect to its power consumption and accuracy.
Investigating the Impact of CNN Depth on Neonatal Seizure Detection Performance
Jun 08, 2018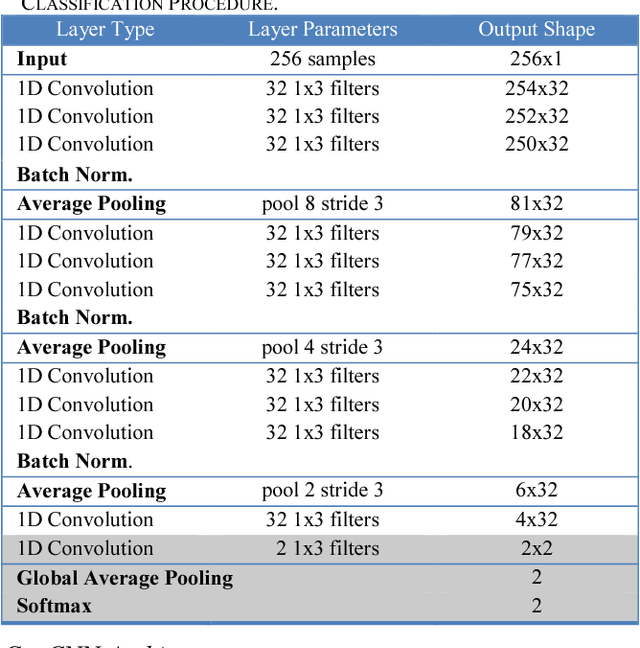
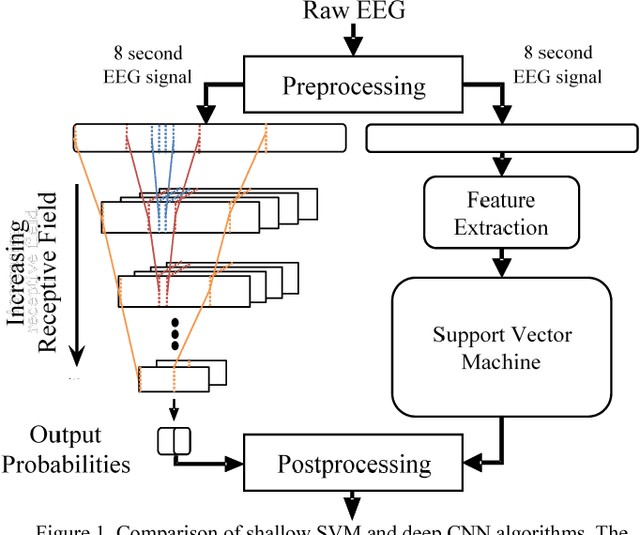
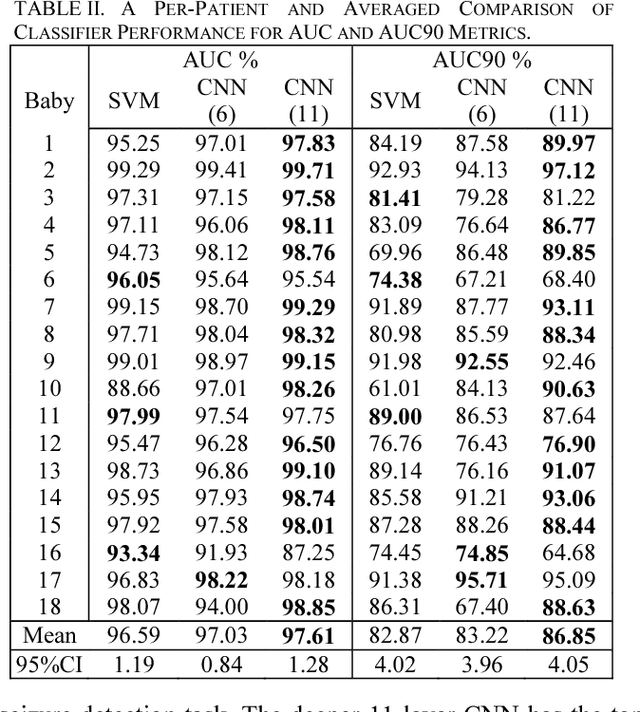
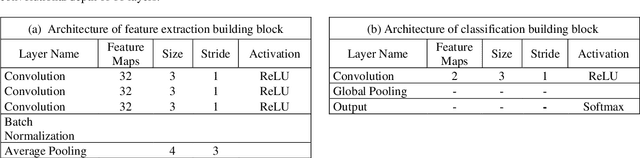
Abstract:This study presents a novel, deep, fully convolutional architecture which is optimized for the task of EEG-based neonatal seizure detection. Architectures of different depths were designed and tested; varying network depth impacts convolutional receptive fields and the corresponding learned feature complexity. Two deep convolutional networks are compared with a shallow SVM-based neonatal seizure detector, which relies on the extraction of hand-crafted features. On a large clinical dataset, of over 800 hours of multichannel unedited EEG, containing 1389 seizure events, the deep 11-layer architecture significantly outperforms the shallower architectures, improving the AUC90 from 82.6% to 86.8%. Combining the end-to-end deep architecture with the feature-based shallow SVM further improves the AUC90 to 87.6%. The fusion of classifiers of different depths gives greatly improved performance and reduced variability, making the combined classifier more clinically reliable.
Neonatal Seizure Detection using Convolutional Neural Networks
Sep 18, 2017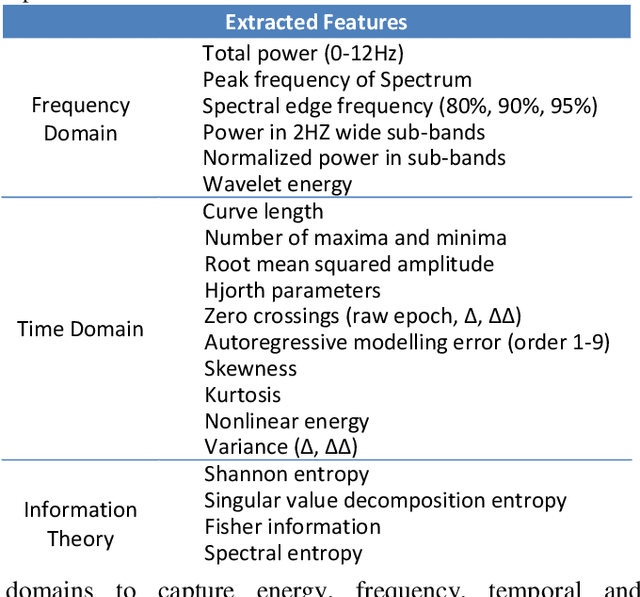
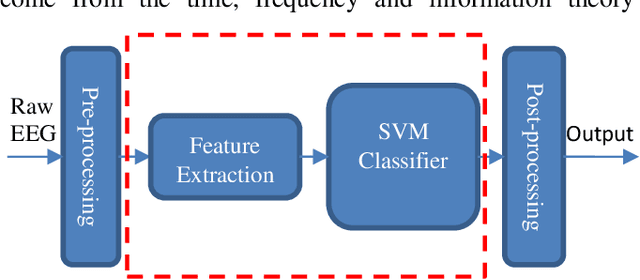
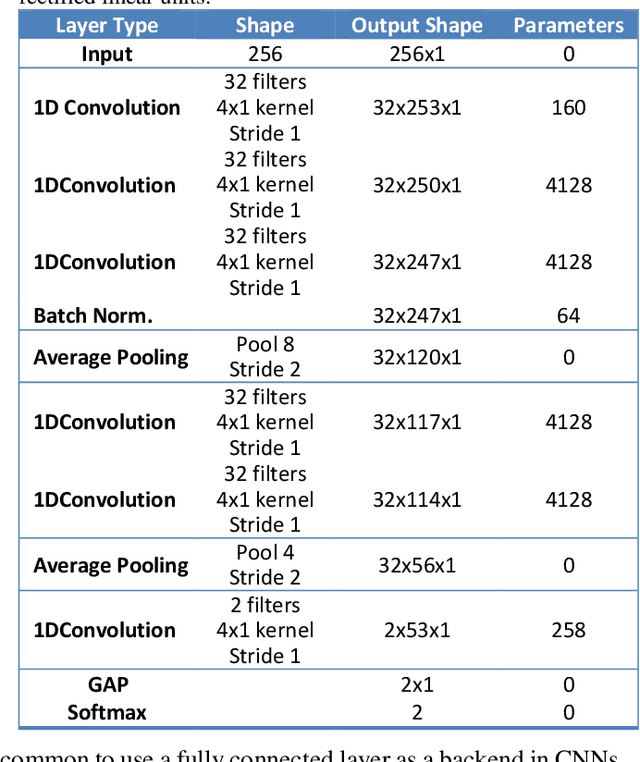
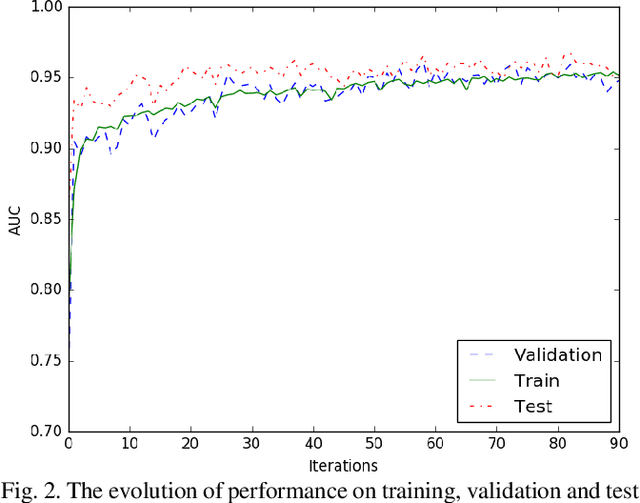
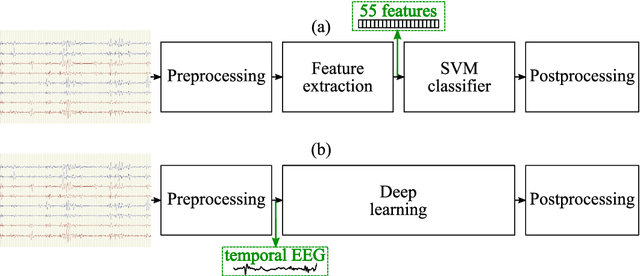
Abstract:This study presents a novel end-to-end architecture that learns hierarchical representations from raw EEG data using fully convolutional deep neural networks for the task of neonatal seizure detection. The deep neural network acts as both feature extractor and classifier, allowing for end-to-end optimization of the seizure detector. The designed system is evaluated on a large dataset of continuous unedited multi-channel neonatal EEG totaling 835 hours and comprising of 1389 seizures. The proposed deep architecture, with sample-level filters, achieves an accuracy that is comparable to the state-of-the-art SVM-based neonatal seizure detector, which operates on a set of carefully designed hand-crafted features. The fully convolutional architecture allows for the localization of EEG waveforms and patterns that result in high seizure probabilities for further clinical examination.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge