Aixa X. Andrade
Mixed Effects Deep Learning for the interpretable analysis of single cell RNA sequencing data by quantifying and visualizing batch effects
Nov 13, 2024
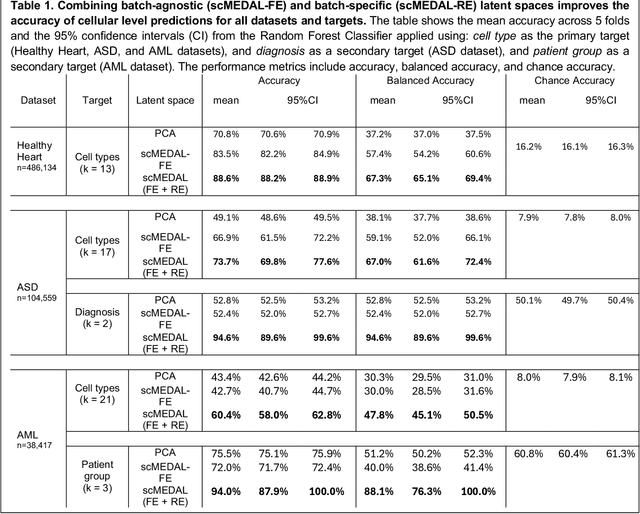


Abstract:Single-cell RNA sequencing (scRNA-seq) data are often confounded by technical or biological batch effects. Existing deep learning models mitigate these effects but often discard batch-specific information, potentially losing valuable biological insights. We propose a Mixed Effects Deep Learning (MEDL) autoencoder framework that separately models batch-invariant (fixed effects) and batch-specific (random effects) components. By decoupling batch-invariant biological states from batch variations, our framework integrates both into predictive models. Our approach also generates 2D visualizations of how the same cell appears across batches, enhancing interpretability. Retaining both fixed and random effect latent spaces improves classification accuracy. We applied our framework to three datasets spanning the cardiovascular system (Healthy Heart), Autism Spectrum Disorder (ASD), and Acute Myeloid Leukemia (AML). With 147 batches in the Healthy Heart dataset, far exceeding typical numbers, we tested our framework's ability to handle many batches. In the ASD dataset, our approach captured donor heterogeneity between autistic and healthy individuals. In the AML dataset, it distinguished donor heterogeneity despite missing cell types and diseased donors exhibiting both healthy and malignant cells. These results highlight our framework's ability to characterize fixed and random effects, enhance batch effect visualization, and improve prediction accuracy across diverse datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge