Optimization of Ensemble Supervised Learning Algorithms for Increased Sensitivity, Specificity, and AUC of Population-Based Colorectal Cancer Screenings
Paper and Code
Aug 15, 2017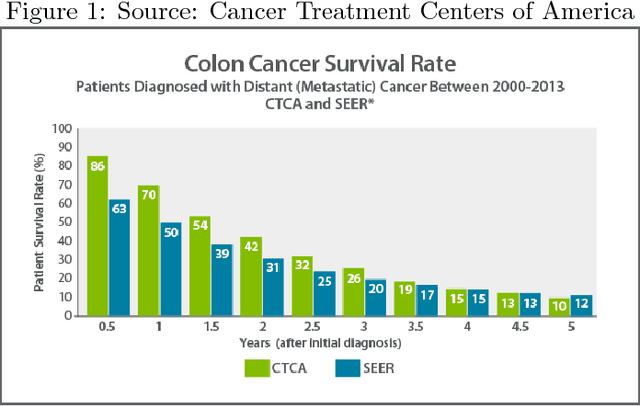
Over 150,000 new people in the United States are diagnosed with colorectal cancer each year. Nearly a third die from it (American Cancer Society). The only approved noninvasive diagnosis tools currently involve fecal blood count tests (FOBTs) or stool DNA tests. Fecal blood count tests take only five minutes and are available over the counter for as low as \$15. They are highly specific, yet not nearly as sensitive, yielding a high percentage (25%) of false negatives (Colon Cancer Alliance). Moreover, FOBT results are far too generalized, meaning that a positive result could mean much more than just colorectal cancer, and could just as easily mean hemorrhoids, anal fissure, proctitis, Crohn's disease, diverticulosis, ulcerative colitis, rectal ulcer, rectal prolapse, ischemic colitis, angiodysplasia, rectal trauma, proctitis from radiation therapy, and others. Stool DNA tests, the modern benchmark for CRC screening, have a much higher sensitivity and specificity, but also cost \$600, take two weeks to process, and are not for high-risk individuals or people with a history of polyps. To yield a cheap and effective CRC screening alternative, a unique ensemble-based classification algorithm is put in place that considers the FIT result, BMI, smoking history, and diabetic status of patients. This method is tested under ten-fold cross validation to have a .95 AUC, 92% specificity, 89% sensitivity, .88 F1, and 90% precision. Once clinically validated, this test promises to be cheaper, faster, and potentially more accurate when compared to a stool DNA test.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge