Neural networks for the prediction organic chemistry reactions
Paper and Code
Oct 17, 2016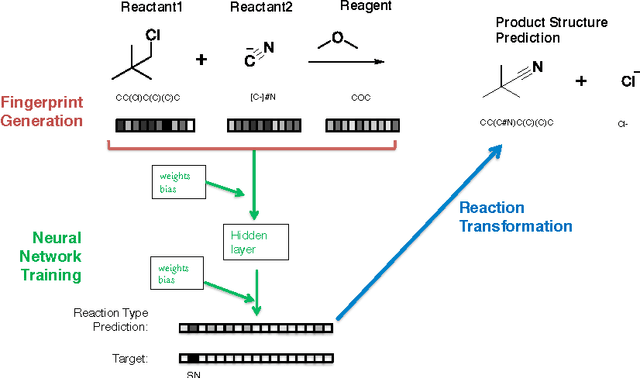

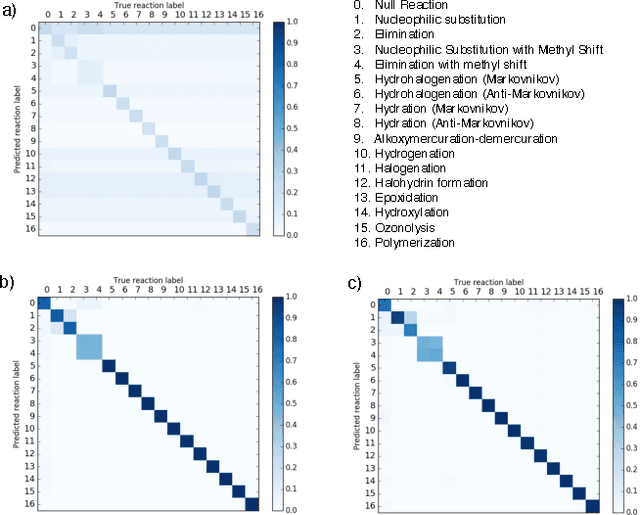
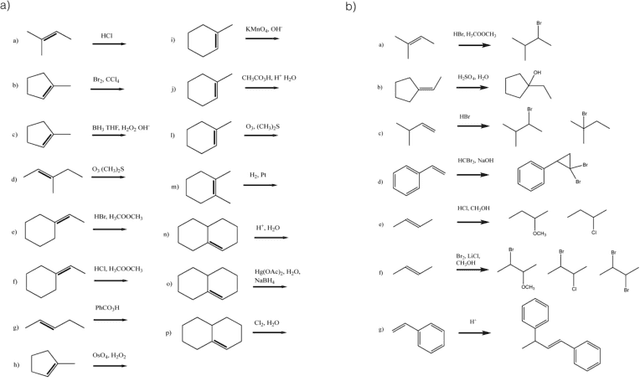
Reaction prediction remains one of the major challenges for organic chemistry, and is a pre-requisite for efficient synthetic planning. It is desirable to develop algorithms that, like humans, "learn" from being exposed to examples of the application of the rules of organic chemistry. We explore the use of neural networks for predicting reaction types, using a new reaction fingerprinting method. We combine this predictor with SMARTS transformations to build a system which, given a set of reagents and re- actants, predicts the likely products. We test this method on problems from a popular organic chemistry textbook.
* ACS.Cent.Sci. 2 (2016) 725-732 * 21 pages, 5 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge