Lipophilicity Prediction with Multitask Learning and Molecular Substructures Representation
Paper and Code
Nov 24, 2020


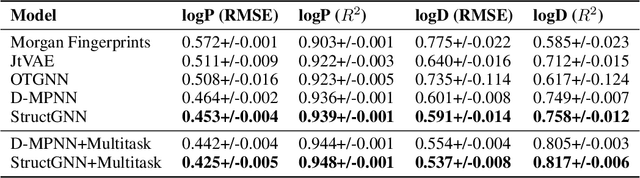
Lipophilicity is one of the factors determining the permeability of the cell membrane to a drug molecule. Hence, accurate lipophilicity prediction is an essential step in the development of new drugs. In this paper, we introduce a novel approach to encoding additional graph information by extracting molecular substructures. By adding a set of generalized atomic features of these substructures to an established Direct Message Passing Neural Network (D-MPNN) we were able to achieve a new state-of-the-art result at the task of prediction of two main lipophilicity coefficients, namely logP and logD descriptors. We further improve our approach by employing a multitask approach to predict logP and logD values simultaneously. Additionally, we present a study of the model performance on symmetric and asymmetric molecules, that may yield insight for further research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge