Guided Multi-objective Generative AI to Enhance Structure-based Drug Design
Paper and Code
May 20, 2024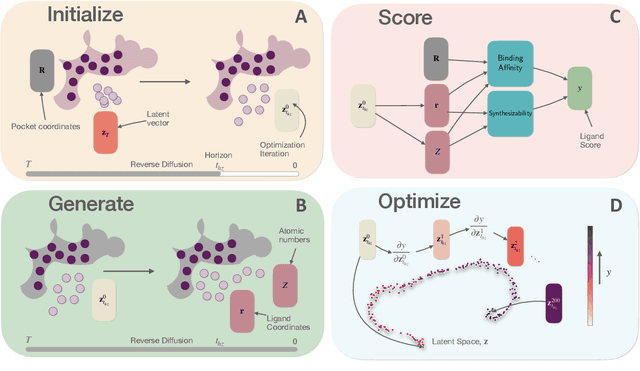

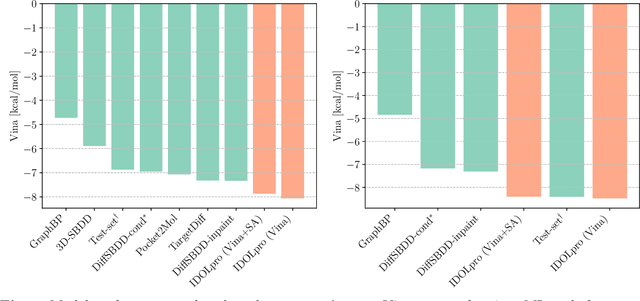
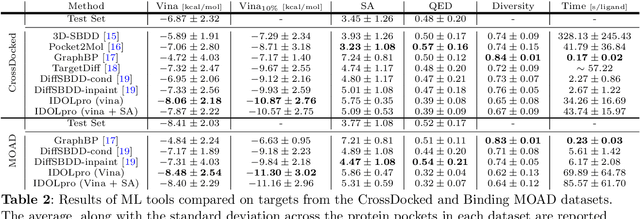
Generative AI has the potential to revolutionize drug discovery. Yet, despite recent advances in machine learning, existing models cannot generate molecules that satisfy all desired physicochemical properties. Herein, we describe IDOLpro, a novel generative chemistry AI combining deep diffusion with multi-objective optimization for structure-based drug design. The latent variables of the diffusion model are guided by differentiable scoring functions to explore uncharted chemical space and generate novel ligands in silico, optimizing a plurality of target physicochemical properties. We demonstrate its effectiveness by generating ligands with optimized binding affinity and synthetic accessibility on two benchmark sets. IDOLpro produces ligands with binding affinities over 10% higher than the next best state-of-the-art on each test set. On a test set of experimental complexes, IDOLpro is the first to surpass the performance of experimentally observed ligands. IDOLpro can accommodate other scoring functions (e.g. ADME-Tox) to accelerate hit-finding, hit-to-lead, and lead optimization for drug discovery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge