Deep-learning models improve on community-level diagnosis for common congenital heart disease lesions
Paper and Code
Sep 19, 2018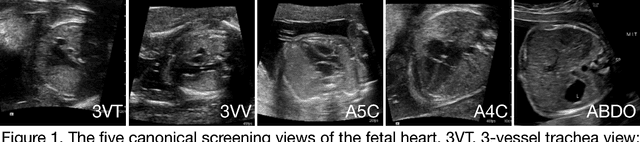
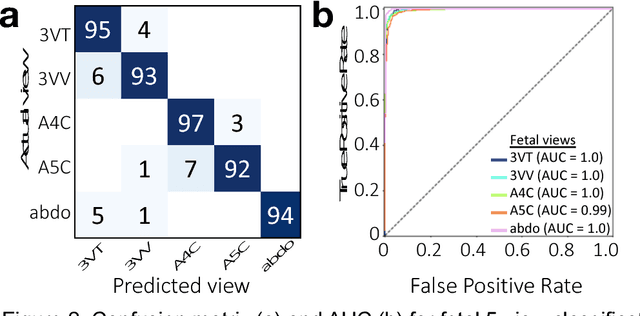

Prenatal diagnosis of tetralogy of Fallot (TOF) and hypoplastic left heart syndrome (HLHS), two serious congenital heart defects, improves outcomes and can in some cases facilitate in utero interventions. In practice, however, the fetal diagnosis rate for these lesions is only 30-50 percent in community settings. Improving fetal diagnosis of congenital heart disease is therefore critical. Deep learning is a cutting-edge machine learning technique for finding patterns in images but has not yet been applied to prenatal diagnosis of congenital heart disease. Using 685 retrospectively collected echocardiograms from fetuses 18-24 weeks of gestational age from 2000-2018, we trained convolutional and fully-convolutional deep learning models in a supervised manner to (i) identify the five canonical screening views of the fetal heart and (ii) segment cardiac structures to calculate fetal cardiac biometrics. We then trained models to distinguish by view between normal hearts, TOF, and HLHS. In a holdout test set of images, F-score for identification of the five most important fetal cardiac views was 0.95. Binary classification of unannotated cardiac views of normal heart vs. TOF reached an overall sensitivity of 75% and a specificity of 76%, while normal vs. HLHS reached a sensitivity of 100% and specificity of 90%, both well above average diagnostic rates for these lesions. Furthermore, segmentation-based measurements for cardiothoracic ratio (CTR), cardiac axis (CA), and ventricular fractional area change (FAC) were compatible with clinically measured metrics for normal, TOF, and HLHS hearts. Thus, using guideline-recommended imaging, deep learning models can significantly improve detection of fetal congenital heart disease compared to the common standard of care.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge