A comment-driven evidence appraisal approach for decision-making when only uncertain evidence available
Paper and Code
Dec 21, 2021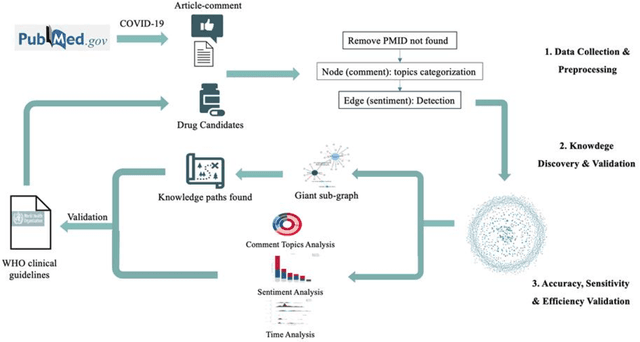

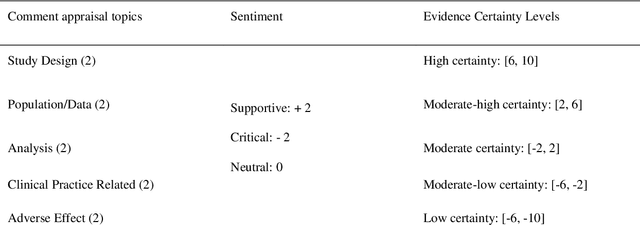

Purpose: To explore whether comments could be used as an assistant tool for heuristic decision-making, especially in cases where missing, incomplete, uncertain, or even incorrect evidence is acquired. Methods: Six COVID-19 drug candidates were selected from WHO clinical guidelines. Evidence-comment networks (ECNs) were completed of these six drug candidates based on evidence-comment pairs from all PubMed indexed COVID-19 publications with formal published comments. WHO guidelines were utilized to validate the feasibility of comment-derived evidence assertions as a fast decision supporting tool. Results: Out of 6 drug candidates, comment-derived evidence assertions of leading subgraphs of 5 drugs were consistent with WHO guidelines, and the overall comment sentiment of 6 drugs was aligned with WHO clinical guidelines. Additionally, comment topics were in accordance with the concerns of guidelines and evidence appraisal criteria. Furthermore, half of the critical comments emerged 4.5 months earlier than the date guidelines were published. Conclusions: Comment-derived evidence assertions have the potential as an evidence appraisal tool for heuristic decisions based on the accuracy, sensitivity, and efficiency of evidence-comment networks. In essence, comments reflect that academic communities do have a self-screening evaluation and self-purification (argumentation) mechanism, thus providing a tool for decision makers to filter evidence.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge