Zach Njus
Flexible and disposable paper- and plastic-based gel micropads for nematode handling, imaging, and chemical testing
Jun 23, 2022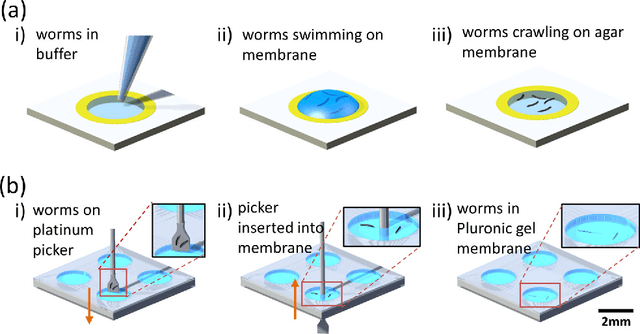
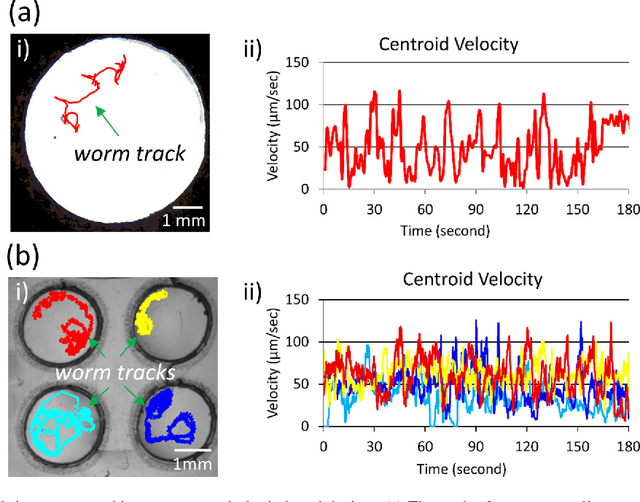
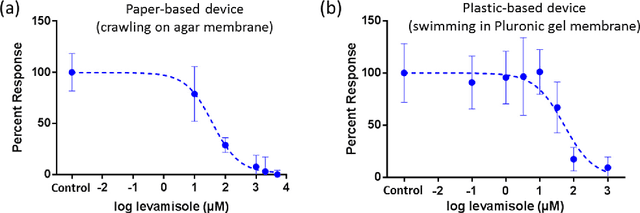
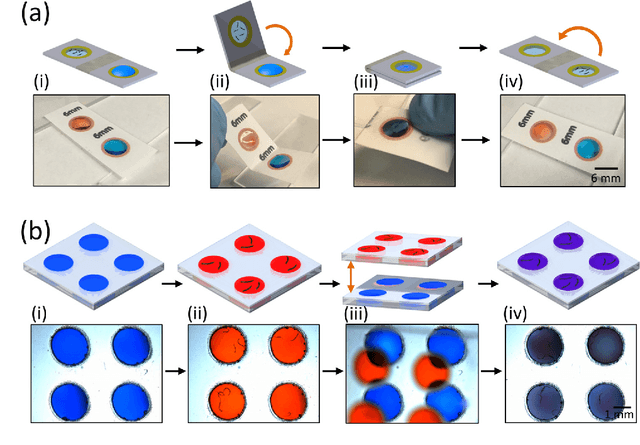
Abstract:Today, the area of point-of-care diagnostics is synonymous with paper microfluidics where cheap, disposable, and on-the-spot detection toolkits are being developed for a variety of chemical tests. In this work, we present a novel application of microfluidic paper-based analytical devices (microPADs) to study the behavior of a small model nematode, Caenorhabditis elegans. We describe schemes of microPAD fabrication on paper and plastic substrates where membranes are created in agarose and Pluronic gel. Methods are demonstrated for loading, visualizing, and transferring single and multiple nematodes. Using an anthelmintic drug, levamisole, we show that chemical testing on C. elegans is easily performed because of the open device structure. A custom program is written to automatically recognize individual worms on the microPADs and extract locomotion parameters in real-time. The combination of microPADs and the nematode tracking program provides a relatively low-cost, simple-to-fabricate imaging and screening assay (compared to standard agarose plates or polymeric microfluidic devices) for non-microfluidic, nematode laboratories.
Effective drug combination for Caenorhabditis elegans nematodes discovered by output-driven feedback system control technique
May 25, 2022Abstract:Infections from parasitic nematodes (or roundworms) contribute to a significant disease burden and productivity losses for humans and livestock. The limited number of anthelmintics (or antinematode drugs) available today to treat these infections are rapidly losing their efficacy as multidrug resistance in parasites becomes a global health challenge. We propose an engineering approach to discover an anthelmintic drug combination that is more potent at killing wild-type Caenorhabditis elegans worms than four individual drugs. In the experiment, freely swimming single worms are enclosed in microfluidic drug environments to assess the centroid velocity and track curvature of worm movements. After analyzing the behavioral data in every iteration, the feedback system control (FSC) scheme is used to predict new drug combinations to test. Through a differential evolutionary search, the winning drug combination is reached that produces minimal centroid velocity and high track curvature, while requiring each drug in less than their EC50 concentrations. The FSC approach is model-less and does not need any information on the drug pharmacology, signaling pathways, or animal biology. Toward combating multidrug resistance, the method presented here is applicable to the discovery of new potent combinations of available anthelmintics on C. elegans, parasitic nematodes, and other small model organisms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge