Vardaan Kishore Kumar
Deep Learning Derived Histopathology Image Score for Increasing Phase 3 Clinical Trial Probability of Success
Nov 10, 2020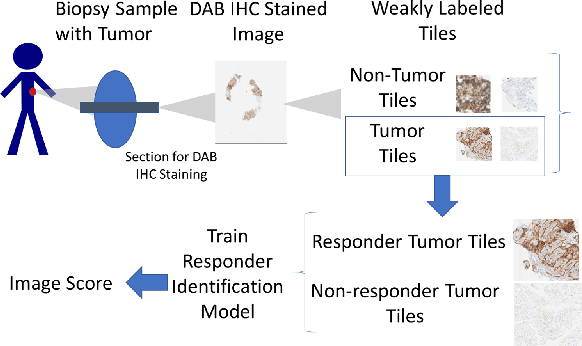
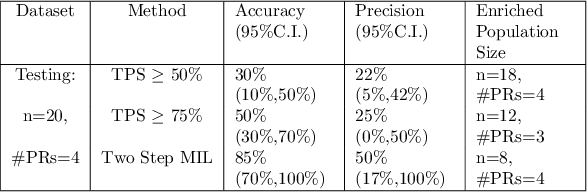
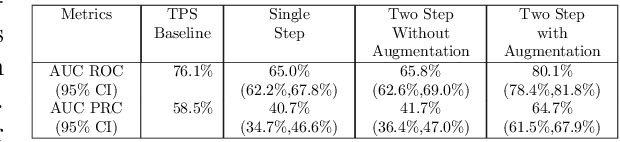
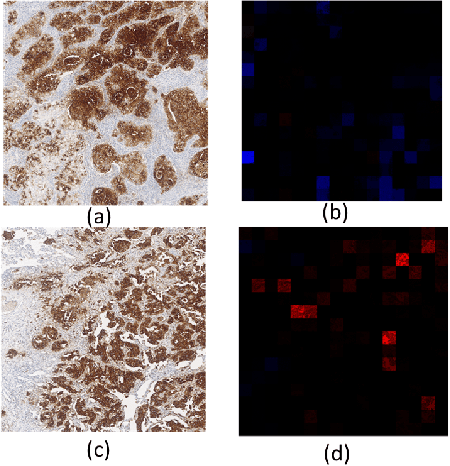
Abstract:Failures in Phase 3 clinical trials contribute to expensive cost of drug development in oncology. To drastically reduce such cost, responders to an oncology treatment need to be identified early on in the drug development process with limited amount of patient data before the planning of Phase 3 clinical trials. Despite the challenge of small sample size, we pioneered the use of deep-learning derived digital pathology scores to identify responders based on the immunohistochemistry images of the target antigen expressed in tumor biopsy samples from a Phase 1 Non-small Cell Lung Cancer clinical trial. Based on repeated 10-fold cross validations, the deep-learning derived score on average achieved 4% higher AUC of ROC curve and 6% higher AUC of Precision-Recall curve comparing to the tumor proportion score (TPS) based clinical benchmark. In a small independent testing set of patients, we also demonstrated that the deep-learning derived score achieved numerically at least 25% higher responder rate in the enriched population than the TPS clinical benchmark.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge