Tanya Wasserman
Symbiotic Message Passing Model for Transfer Learning between Anti-Fungal and Anti-Bacterial Domains
Apr 14, 2023



Abstract:Machine learning, and representation learning in particular, has the potential to facilitate drug discovery by screening billions of compounds. For example, a successful approach is representing the molecules as a graph and utilizing graph neural networks (GNN). Yet, these approaches still require experimental measurements of thousands of compounds to construct a proper training set. While in some domains it is easier to acquire experimental data, in others it might be more limited. For example, it is easier to test the compounds on bacteria than perform in-vivo experiments. Thus, a key question is how to utilize information from a large available dataset together with a small subset of compounds where both domains are measured to predict compounds' effect on the second, experimentally less available domain. Current transfer learning approaches for drug discovery, including training of pre-trained modules or meta-learning, have limited success. In this work, we develop a novel method, named Symbiotic Message Passing Neural Network (SMPNN), for merging graph-neural-network models from different domains. Using routing new message passing lanes between them, our approach resolves some of the potential conflicts between the different domains, and implicit constraints induced by the larger datasets. By collecting public data and performing additional high-throughput experiments, we demonstrate the advantage of our approach by predicting anti-fungal activity from anti-bacterial activity. We compare our method to the standard transfer learning approach and show that SMPNN provided better and less variable performances. Our approach is general and can be used to facilitate information transfer between any two domains such as different organisms, different organelles, or different environments.
Harnessing Artificial Intelligence to Infer Novel Spatial Biomarkers for the Diagnosis of Eosinophilic Esophagitis
May 26, 2022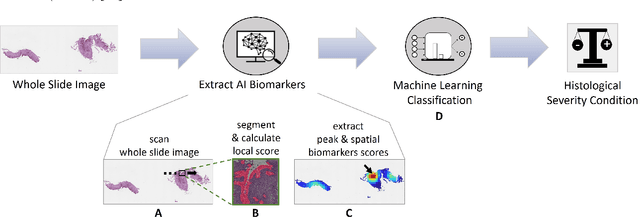
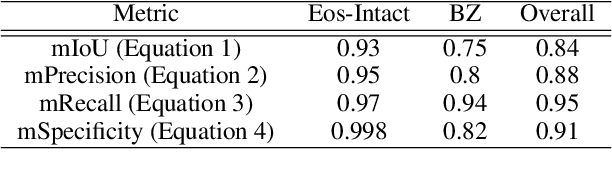
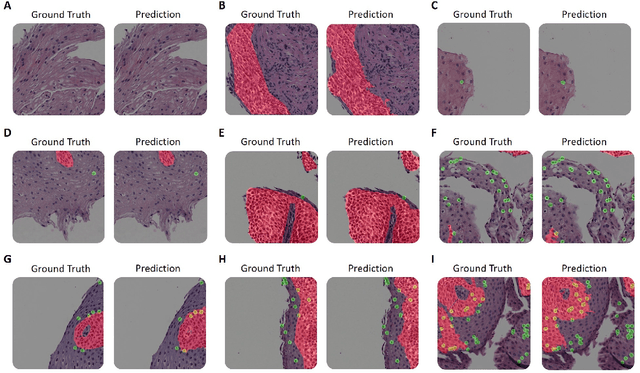
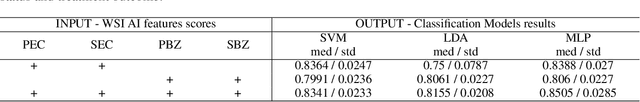
Abstract:Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory condition of the esophagus associated with elevated esophageal eosinophils. Second only to gastroesophageal reflux disease, EoE is one of the leading causes of chronic refractory dysphagia in adults and children. EoE diagnosis requires enumerating the density of esophageal eosinophils in esophageal biopsies, a somewhat subjective task that is time-consuming, thus reducing the ability to process the complex tissue structure. Previous artificial intelligence (AI) approaches that aimed to improve histology-based diagnosis focused on recapitulating identification and quantification of the area of maximal eosinophil density. However, this metric does not account for the distribution of eosinophils or other histological features, over the whole slide image. Here, we developed an artificial intelligence platform that infers local and spatial biomarkers based on semantic segmentation of intact eosinophils and basal zone distributions. Besides the maximal density of eosinophils (referred to as Peak Eosinophil Count [PEC]) and a maximal basal zone fraction, we identify two additional metrics that reflect the distribution of eosinophils and basal zone fractions. This approach enables a decision support system that predicts EoE activity and classifies the histological severity of EoE patients. We utilized a cohort that includes 1066 biopsy slides from 400 subjects to validate the system's performance and achieved a histological severity classification accuracy of 86.70%, sensitivity of 84.50%, and specificity of 90.09%. Our approach highlights the importance of systematically analyzing the distribution of biopsy features over the entire slide and paves the way towards a personalized decision support system that will assist not only in counting cells but can also potentially improve diagnosis and provide treatment prediction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge