Shashank Acharya
Esophageal virtual disease landscape using mechanics-informed machine learning
Nov 19, 2021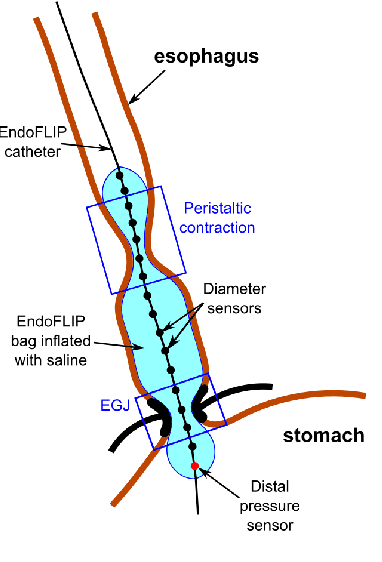
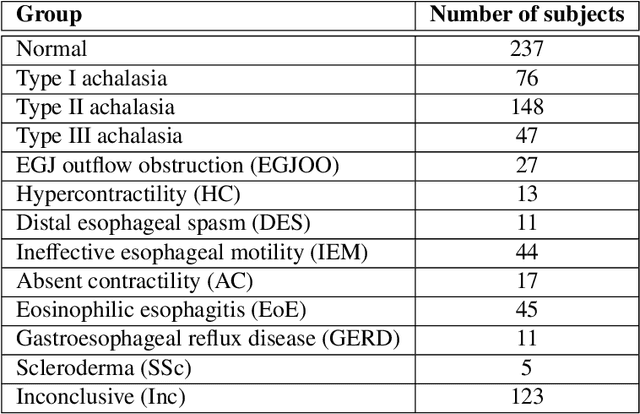
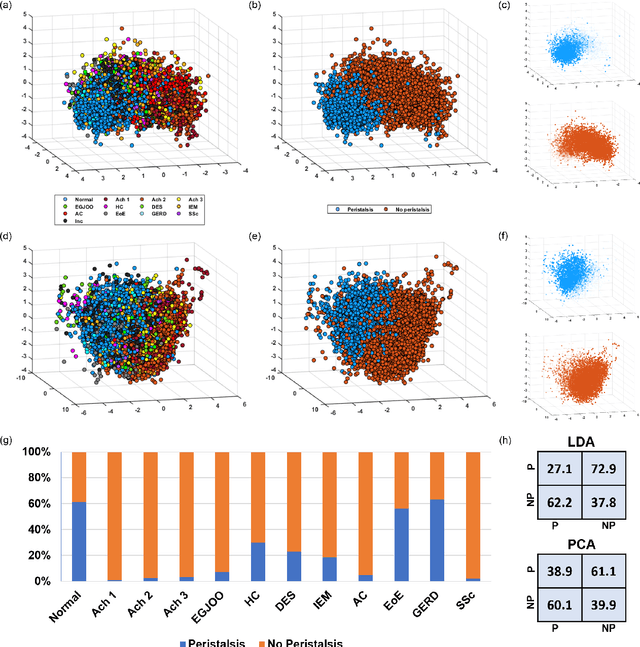
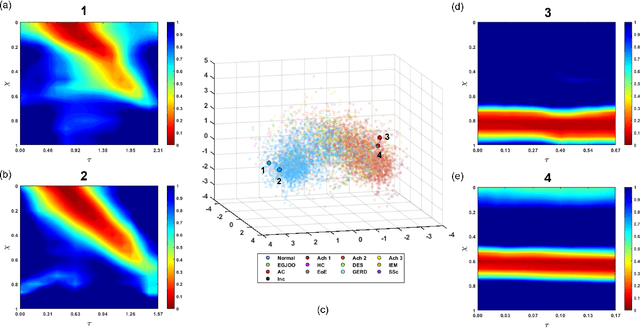
Abstract:The pathogenesis of esophageal disorders is related to the esophageal wall mechanics. Therefore, to understand the underlying fundamental mechanisms behind various esophageal disorders, it is crucial to map the esophageal wall mechanics-based parameters onto physiological and pathophysiological conditions corresponding to altered bolus transit and supraphysiologic IBP. In this work, we present a hybrid framework that combines fluid mechanics and machine learning to identify the underlying physics of the various esophageal disorders and maps them onto a parameter space which we call the virtual disease landscape (VDL). A one-dimensional inverse model processes the output from an esophageal diagnostic device called endoscopic functional lumen imaging probe (EndoFLIP) to estimate the mechanical "health" of the esophagus by predicting a set of mechanics-based parameters such as esophageal wall stiffness, muscle contraction pattern and active relaxation of esophageal walls. The mechanics-based parameters were then used to train a neural network that consists of a variational autoencoder (VAE) that generates a latent space and a side network that predicts mechanical work metrics for estimating esophagogastric junction motility. The latent vectors along with a set of discrete mechanics-based parameters define the VDL and form clusters corresponding to the various esophageal disorders. The VDL not only distinguishes different disorders but can also be used to predict disease progression in time. Finally, we also demonstrate the clinical applicability of this framework for estimating the effectiveness of a treatment and track patient condition after a treatment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge