John E. Pandolfino
MRI-MECH: Mechanics-informed MRI to estimate esophageal health
Sep 15, 2022



Abstract:Dynamic magnetic resonance imaging (MRI) is a popular medical imaging technique to generate image sequences of the flow of a contrast material inside tissues and organs. However, its application to imaging bolus movement through the esophagus has only been demonstrated in few feasibility studies and is relatively unexplored. In this work, we present a computational framework called mechanics-informed MRI (MRI-MECH) that enhances that capability thereby increasing the applicability of dynamic MRI for diagnosing esophageal disorders. Pineapple juice was used as the swallowed contrast material for the dynamic MRI and the MRI image sequence was used as input to the MRI-MECH. The MRI-MECH modeled the esophagus as a flexible one-dimensional tube and the elastic tube walls followed a linear tube law. Flow through the esophagus was then governed by one-dimensional mass and momentum conservation equations. These equations were solved using a physics-informed neural network (PINN). The PINN minimized the difference between the measurements from the MRI and model predictions ensuring that the physics of the fluid flow problem was always followed. MRI-MECH calculated the fluid velocity and pressure during esophageal transit and estimated the mechanical health of the esophagus by calculating wall stiffness and active relaxation. Additionally, MRI-MECH predicted missing information about the lower esophageal sphincter during the emptying process, demonstrating its applicability to scenarios with missing data or poor image resolution. In addition to potentially improving clinical decisions based on quantitative estimates of the mechanical health of the esophagus, MRI-MECH can also be enhanced for application to other medical imaging modalities to enhance their functionality as well.
Esophageal virtual disease landscape using mechanics-informed machine learning
Nov 19, 2021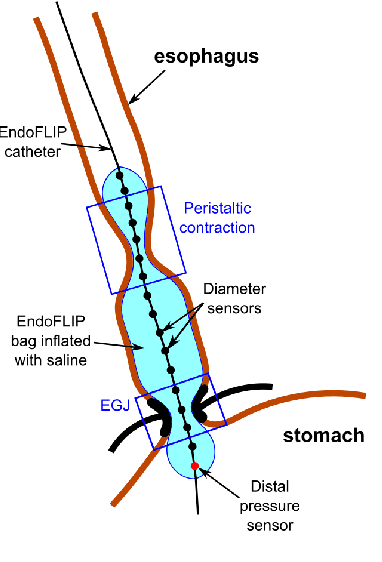
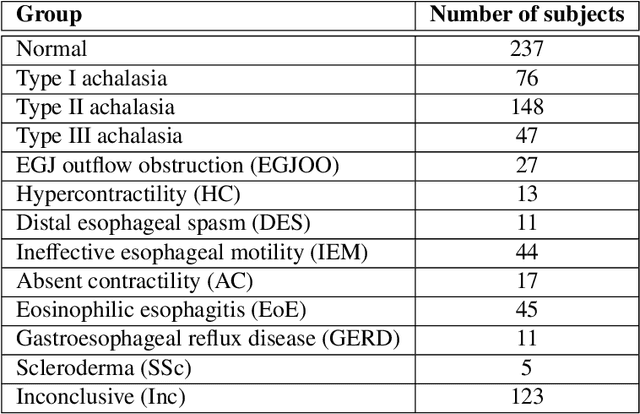
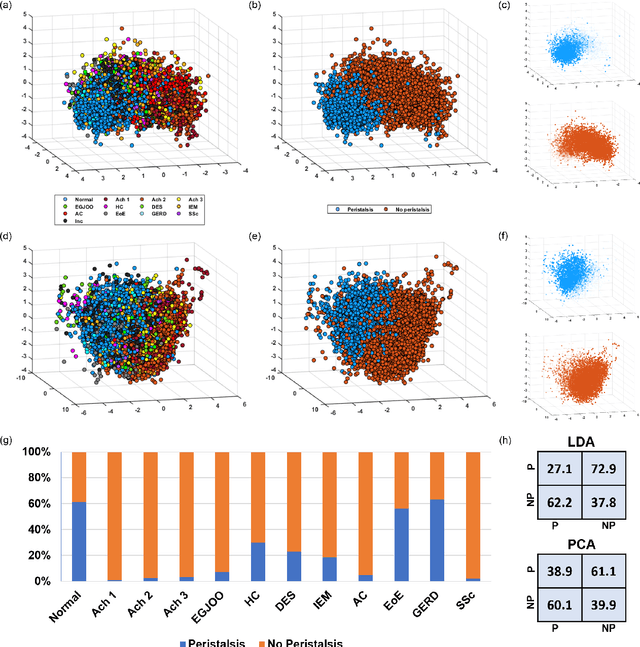
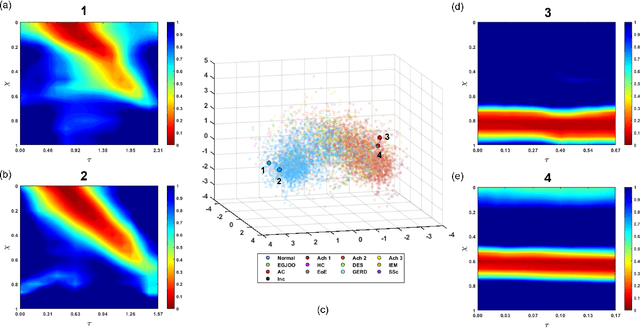
Abstract:The pathogenesis of esophageal disorders is related to the esophageal wall mechanics. Therefore, to understand the underlying fundamental mechanisms behind various esophageal disorders, it is crucial to map the esophageal wall mechanics-based parameters onto physiological and pathophysiological conditions corresponding to altered bolus transit and supraphysiologic IBP. In this work, we present a hybrid framework that combines fluid mechanics and machine learning to identify the underlying physics of the various esophageal disorders and maps them onto a parameter space which we call the virtual disease landscape (VDL). A one-dimensional inverse model processes the output from an esophageal diagnostic device called endoscopic functional lumen imaging probe (EndoFLIP) to estimate the mechanical "health" of the esophagus by predicting a set of mechanics-based parameters such as esophageal wall stiffness, muscle contraction pattern and active relaxation of esophageal walls. The mechanics-based parameters were then used to train a neural network that consists of a variational autoencoder (VAE) that generates a latent space and a side network that predicts mechanical work metrics for estimating esophagogastric junction motility. The latent vectors along with a set of discrete mechanics-based parameters define the VDL and form clusters corresponding to the various esophageal disorders. The VDL not only distinguishes different disorders but can also be used to predict disease progression in time. Finally, we also demonstrate the clinical applicability of this framework for estimating the effectiveness of a treatment and track patient condition after a treatment.
A multi-stage machine learning model on diagnosis of esophageal manometry
Jun 25, 2021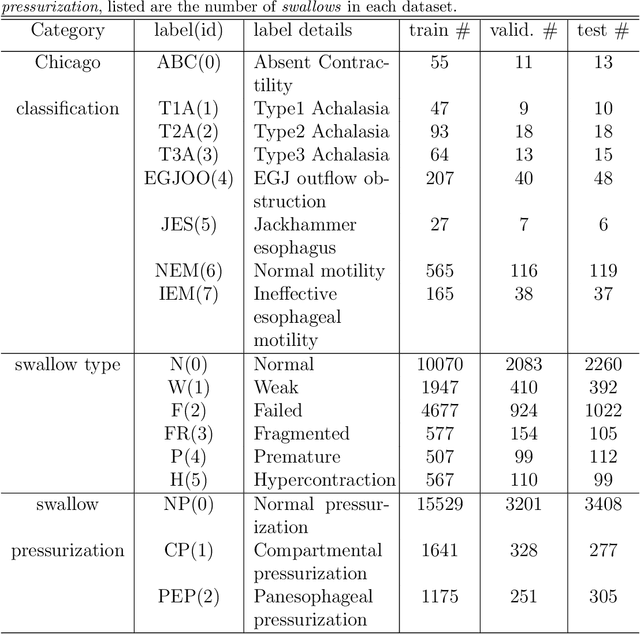
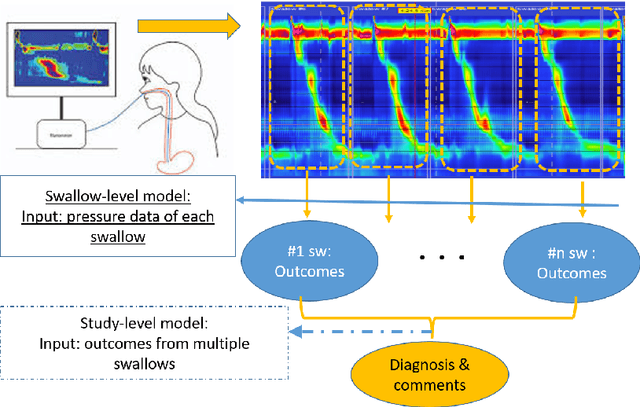
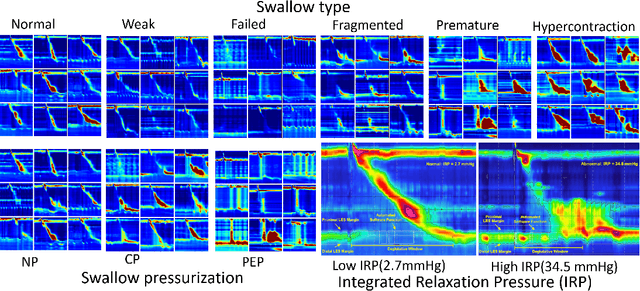
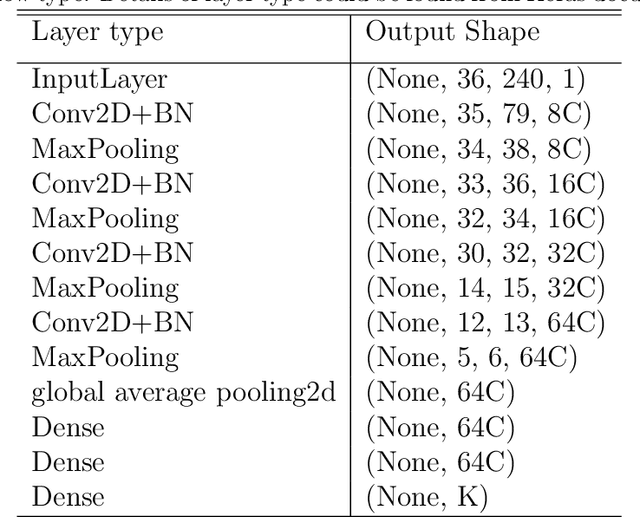
Abstract:High-resolution manometry (HRM) is the primary procedure used to diagnose esophageal motility disorders. Its interpretation and classification includes an initial evaluation of swallow-level outcomes and then derivation of a study-level diagnosis based on Chicago Classification (CC), using a tree-like algorithm. This diagnostic approach on motility disordered using HRM was mirrored using a multi-stage modeling framework developed using a combination of various machine learning approaches. Specifically, the framework includes deep-learning models at the swallow-level stage and feature-based machine learning models at the study-level stage. In the swallow-level stage, three models based on convolutional neural networks (CNNs) were developed to predict swallow type, swallow pressurization, and integrated relaxation pressure (IRP). At the study-level stage, model selection from families of the expert-knowledge-based rule models, xgboost models and artificial neural network(ANN) models were conducted, with the latter two model designed and augmented with motivation from the export knowledge. A simple model-agnostic strategy of model balancing motivated by Bayesian principles was utilized, which gave rise to model averaging weighted by precision scores. The averaged (blended) models and individual models were compared and evaluated, of which the best performance on test dataset is 0.81 in top-1 prediction, 0.92 in top-2 predictions. This is the first artificial-intelligence-style model to automatically predict CC diagnosis of HRM study from raw multi-swallow data. Moreover, the proposed modeling framework could be easily extended to multi-modal tasks, such as diagnosis of esophageal patients based on clinical data from both HRM and functional luminal imaging probe panometry (FLIP).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge