Sean R. Mathieson
Scaling convolutional neural networks achieves expert-level seizure detection in neonatal EEG
May 16, 2024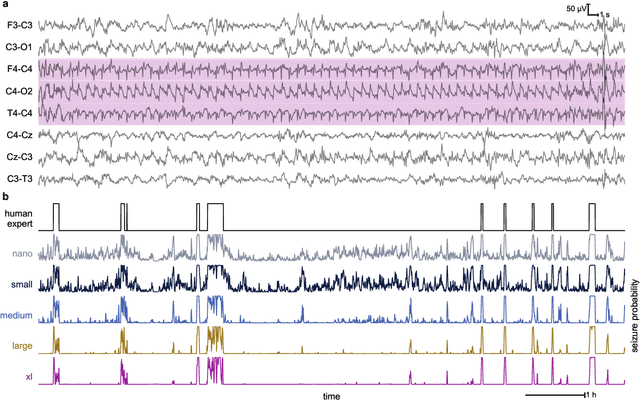
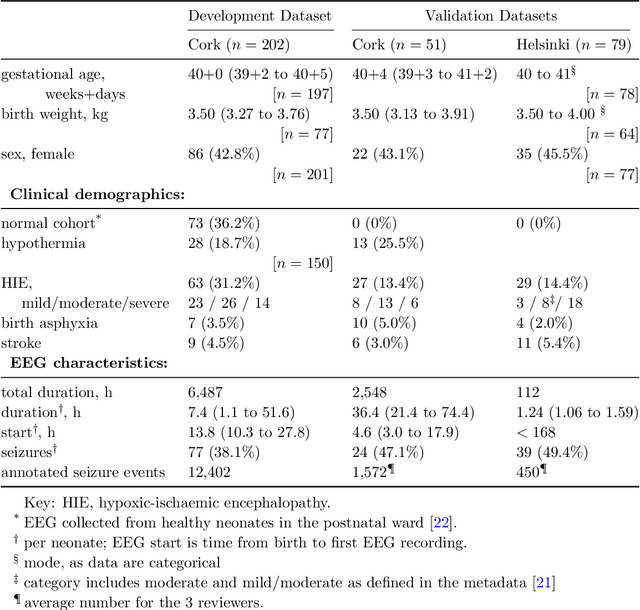
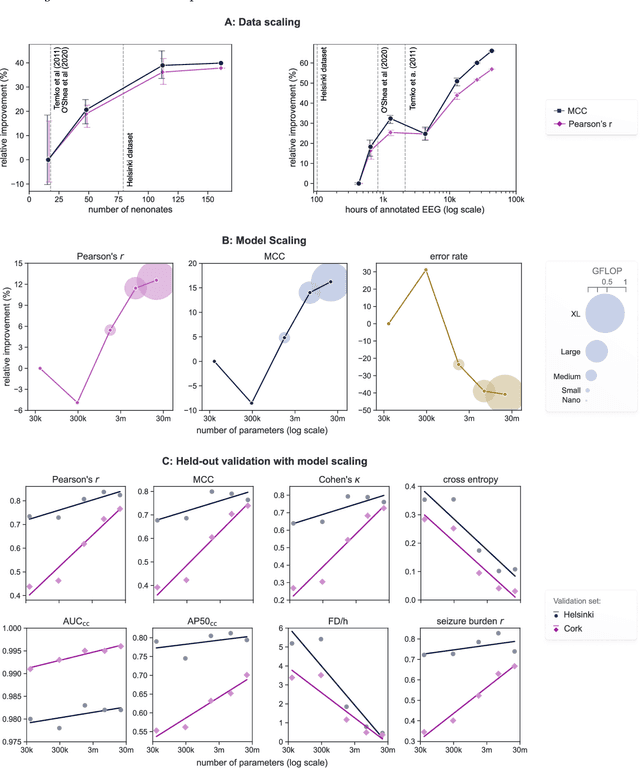
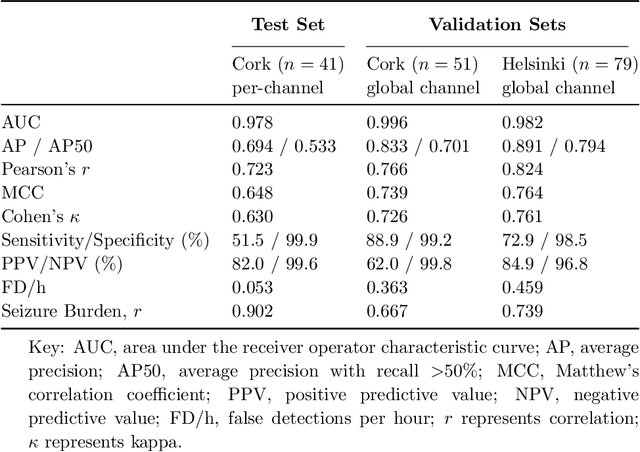
Abstract:Background: Neonatal seizures are a neurological emergency that require urgent treatment. They are hard to diagnose clinically and can go undetected if EEG monitoring is unavailable. EEG interpretation requires specialised expertise which is not widely available. Algorithms to detect EEG seizures can address this limitation but have yet to reach widespread clinical adoption. Methods: Retrospective EEG data from 332 neonates was used to develop and validate a seizure-detection model. The model was trained and tested with a development dataset ($n=202$) that was annotated with over 12k seizure events on a per-channel basis. This dataset was used to develop a convolutional neural network (CNN) using a modern architecture and training methods. The final model was then validated on two independent multi-reviewer datasets ($n=51$ and $n=79$). Results: Increasing dataset and model size improved model performance: Matthews correlation coefficient (MCC) and Pearson's correlation ($r$) increased by up to 50% with data scaling and up to 15% with model scaling. Over 50k hours of annotated single-channel EEG was used for training a model with 21 million parameters. State-of-the-art was achieved on an open-access dataset (MCC=0.764, $r=0.824$, and AUC=0.982). The CNN attains expert-level performance on both held-out validation sets, with no significant difference in inter-rater agreement among the experts and among experts and algorithm ($\Delta \kappa < -0.095$, $p>0.05$). Conclusion: With orders of magnitude increases in data and model scale we have produced a new state-of-the-art model for neonatal seizure detection. Expert-level equivalence on completely unseen data, a first in this field, provides a strong indication that the model is ready for further clinical validation.
Tracé alternant detector for grading hypoxic-ischemic encephalopathy in neonatal EEG
May 31, 2021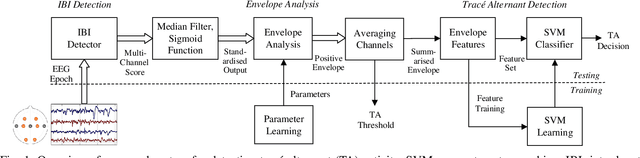
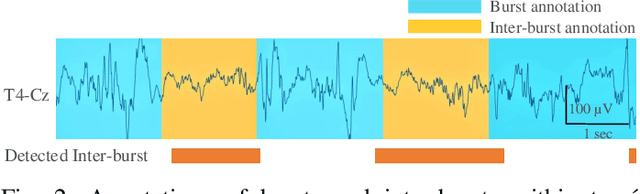
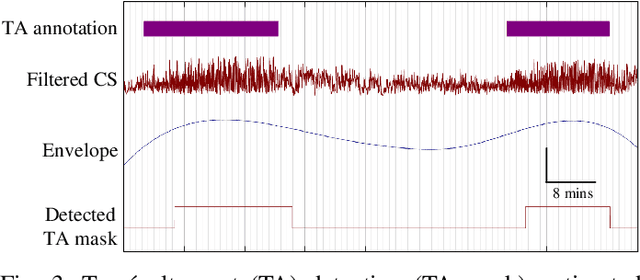
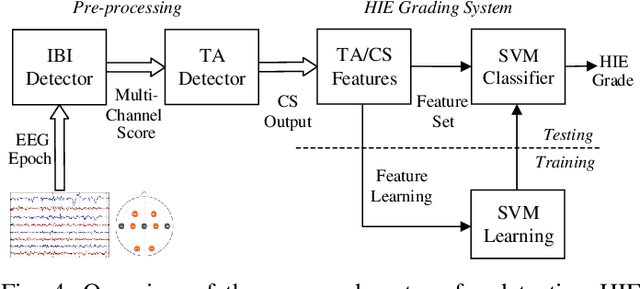
Abstract:Electroencephalography (EEG) is an important clinical tool to capture sleep-wake cycling. It can also be used for grading injury, known as hypoxic-ischaemic encephalopathy(HIE), caused by lack of oxygen or blood to the brain during birth. Trac\'e alternant (TA) is a distinctive component of normal quiet sleep which consists of alternating periods of high-voltage activity (bursts) separated by lower-voltage activity (inter-bursts). This study presents an automated method to grade the severity of injury in HIE, using an automated method to first detect activity. The TA detector uses the output of an existing method to detect inter-bursts. Features are extracted from a processed output and then combined in a support vector machine (SVM). Next, we develop an HIE grading system using the TA detector by combining different features from the temporal organisation of the detected TA mask, again using an SVM. Training and testing for both models use a leave-one-baby-out cross-validation procedure, with model hyper-parameters selected from nested cross validations. The TA detector, tested on EEG from 71 healthy term neonates, has an accuracy of 79.1% (Cohen's \k{appa}=0.55). the grading system, tested on EEG from 54 term neonates in intensive care, has an accuracy of 81.5% (\k{appa}=0.74). These results validate how detecting the presence or absence of TA can be used to quantify the grade of HIE injury in neonatal EEG and open up the possibility of a clinically-meaningful grading system.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge