Sandeep Gupta
Dynamic Hand Gesture Recognition for Robot Manipulator Tasks
Jan 19, 2026Abstract:This paper proposes a novel approach to recognizing dynamic hand gestures facilitating seamless interaction between humans and robots. Here, each robot manipulator task is assigned a specific gesture. There may be several such tasks, hence, several gestures. These gestures may be prone to several dynamic variations. All such variations for different gestures shown to the robot are accurately recognized in real-time using the proposed unsupervised model based on the Gaussian Mixture model. The accuracy during training and real-time testing prove the efficacy of this methodology.
XAI-MeD: Explainable Knowledge Guided Neuro-Symbolic Framework for Domain Generalization and Rare Class Detection in Medical Imaging
Jan 05, 2026Abstract:Explainability domain generalization and rare class reliability are critical challenges in medical AI where deep models often fail under real world distribution shifts and exhibit bias against infrequent clinical conditions This paper introduces XAIMeD an explainable medical AI framework that integrates clinically accurate expert knowledge into deep learning through a unified neuro symbolic architecture XAIMeD is designed to improve robustness under distribution shift enhance rare class sensitivity and deliver transparent clinically aligned interpretations The framework encodes clinical expertise as logical connectives over atomic medical propositions transforming them into machine checkable class specific rules Their diagnostic utility is quantified through weighted feature satisfaction scores enabling a symbolic reasoning branch that complements neural predictions A confidence weighted fusion integrates symbolic and deep outputs while a Hunt inspired adaptive routing mechanism guided by Entropy Imbalance Gain EIG and Rare Class Gini mitigates class imbalance high intra class variability and uncertainty We evaluate XAIMeD across diverse modalities on four challenging tasks i Seizure Onset Zone SOZ localization from rs fMRI ii Diabetic Retinopathy grading across 6 multicenter datasets demonstrate substantial performance improvements including 6 percent gains in cross domain generalization and a 10 percent improved rare class F1 score far outperforming state of the art deep learning baselines Ablation studies confirm that the clinically grounded symbolic components act as effective regularizers ensuring robustness to distribution shifts XAIMeD thus provides a principled clinically faithful and interpretable approach to multimodal medical AI.
Enabling Physical AI at the Edge: Hardware-Accelerated Recovery of System Dynamics
Dec 29, 2025Abstract:Physical AI at the edge -- enabling autonomous systems to understand and predict real-world dynamics in real time -- requires hardware-efficient learning and inference. Model recovery (MR), which identifies governing equations from sensor data, is a key primitive for safe and explainable monitoring in mission-critical autonomous systems operating under strict latency, compute, and power constraints. However, state-of-the-art MR methods (e.g., EMILY and PINN+SR) rely on Neural ODE formulations that require iterative solvers and are difficult to accelerate efficiently on edge hardware. We present \textbf{MERINDA} (Model Recovery in Reconfigurable Dynamic Architecture), an FPGA-accelerated MR framework designed to make physical AI practical on resource-constrained devices. MERINDA replaces expensive Neural ODE components with a hardware-friendly formulation that combines (i) GRU-based discretized dynamics, (ii) dense inverse-ODE layers, (iii) sparsity-driven dropout, and (iv) lightweight ODE solvers. The resulting computation is structured for streaming parallelism, enabling critical kernels to be fully parallelized on the FPGA. Across four benchmark nonlinear dynamical systems, MERINDA delivers substantial gains over GPU implementations: \textbf{114$\times$ lower energy} (434~J vs.\ 49{,}375~J), \textbf{28$\times$ smaller memory footprint} (214~MB vs.\ 6{,}118~MB), and \textbf{1.68$\times$ faster training}, while matching state-of-the-art model-recovery accuracy. These results demonstrate that MERINDA can bring accurate, explainable MR to the edge for real-time monitoring of autonomous systems.
NEURO-GUARD: Neuro-Symbolic Generalization and Unbiased Adaptive Routing for Diagnostics -- Explainable Medical AI
Dec 20, 2025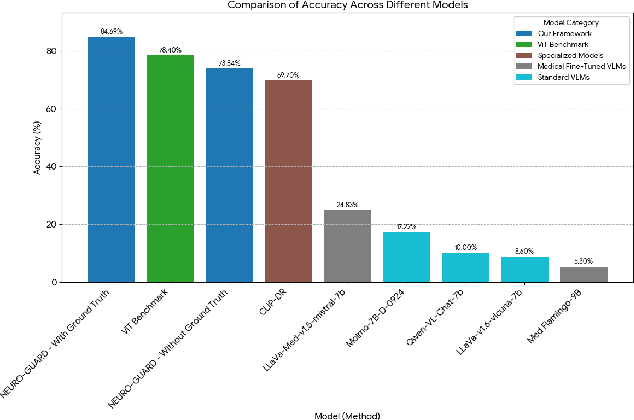
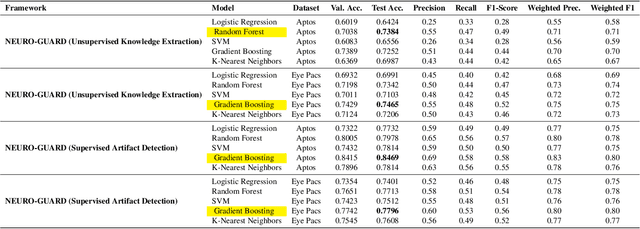
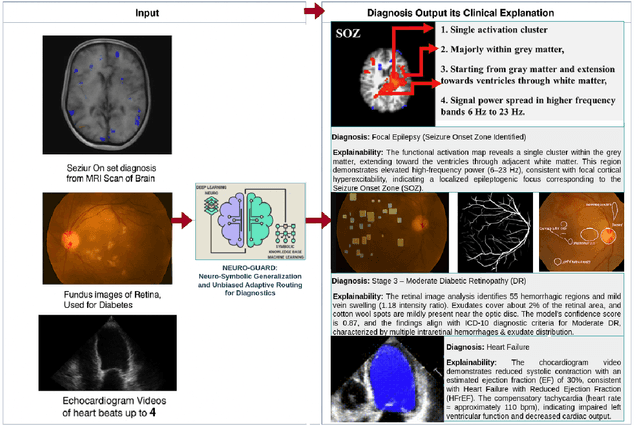
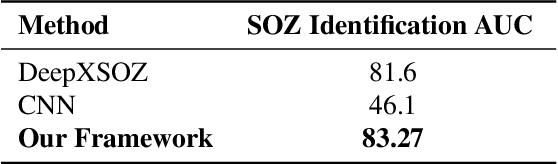
Abstract:Accurate yet interpretable image-based diagnosis remains a central challenge in medical AI, particularly in settings characterized by limited data, subtle visual cues, and high-stakes clinical decision-making. Most existing vision models rely on purely data-driven learning and produce black-box predictions with limited interpretability and poor cross-domain generalization, hindering their real-world clinical adoption. We present NEURO-GUARD, a novel knowledge-guided vision framework that integrates Vision Transformers (ViTs) with language-driven reasoning to improve performance, transparency, and domain robustness. NEURO-GUARD employs a retrieval-augmented generation (RAG) mechanism for self-verification, in which a large language model (LLM) iteratively generates, evaluates, and refines feature-extraction code for medical images. By grounding this process in clinical guidelines and expert knowledge, the framework progressively enhances feature detection and classification beyond purely data-driven baselines. Extensive experiments on diabetic retinopathy classification across four benchmark datasets APTOS, EyePACS, Messidor-1, and Messidor-2 demonstrate that NEURO-GUARD improves accuracy by 6.2% over a ViT-only baseline (84.69% vs. 78.4%) and achieves a 5% gain in domain generalization. Additional evaluations on MRI-based seizure detection further confirm its cross-domain robustness, consistently outperforming existing methods. Overall, NEURO-GUARD bridges symbolic medical reasoning with subsymbolic visual learning, enabling interpretable, knowledge-aware, and generalizable medical image diagnosis while achieving state-of-the-art performance across multiple datasets.
MedXAI: A Retrieval-Augmented and Self-Verifying Framework for Knowledge-Guided Medical Image Analysis
Dec 10, 2025Abstract:Accurate and interpretable image-based diagnosis remains a fundamental challenge in medical AI, particularly under domain shifts and rare-class conditions. Deep learning models often struggle with real-world distribution changes, exhibit bias against infrequent pathologies, and lack the transparency required for deployment in safety-critical clinical environments. We introduce MedXAI (An Explainable Framework for Medical Imaging Classification), a unified expert knowledge based framework that integrates deep vision models with clinician-derived expert knowledge to improve generalization, reduce rare-class bias, and provide human-understandable explanations by localizing the relevant diagnostic features rather than relying on technical post-hoc methods (e.g., Saliency Maps, LIME). We evaluate MedXAI across heterogeneous modalities on two challenging tasks: (i) Seizure Onset Zone localization from resting-state fMRI, and (ii) Diabetic Retinopathy grading. Ex periments on ten multicenter datasets show consistent gains, including a 3% improvement in cross-domain generalization and a 10% improvmnet in F1 score of rare class, substantially outperforming strong deep learning baselines. Ablations confirm that the symbolic components act as effective clinical priors and regularizers, improving robustness under distribution shift. MedXAI delivers clinically aligned explanations while achieving superior in-domain and cross-domain performance, particularly for rare diseases in multimodal medical AI.
Generating customized prompts for Zero-Shot Rare Event Medical Image Classification using LLM
Jan 27, 2025Abstract:Rare events, due to their infrequent occurrences, do not have much data, and hence deep learning techniques fail in estimating the distribution for such data. Open-vocabulary models represent an innovative approach to image classification. Unlike traditional models, these models classify images into any set of categories specified with natural language prompts during inference. These prompts usually comprise manually crafted templates (e.g., 'a photo of a {}') that are filled in with the names of each category. This paper introduces a simple yet effective method for generating highly accurate and contextually descriptive prompts containing discriminative characteristics. Rare event detection, especially in medicine, is more challenging due to low inter-class and high intra-class variability. To address these, we propose a novel approach that uses domain-specific expert knowledge on rare events to generate customized and contextually relevant prompts, which are then used by large language models for image classification. Our zero-shot, privacy-preserving method enhances rare event classification without additional training, outperforming state-of-the-art techniques.
Framework for developing and evaluating ethical collaboration between expert and machine
Nov 17, 2024Abstract:Precision medicine is a promising approach for accessible disease diagnosis and personalized intervention planning in high-mortality diseases such as coronary artery disease (CAD), drug-resistant epilepsy (DRE), and chronic illnesses like Type 1 diabetes (T1D). By leveraging artificial intelligence (AI), precision medicine tailors diagnosis and treatment solutions to individual patients by explicitly modeling variance in pathophysiology. However, the adoption of AI in medical applications faces significant challenges, including poor generalizability across centers, demographics, and comorbidities, limited explainability in clinical terms, and a lack of trust in ethical decision-making. This paper proposes a framework to develop and ethically evaluate expert-guided multi-modal AI, addressing these challenges in AI integration within precision medicine. We illustrate this framework with case study on insulin management for T1D. To ensure ethical considerations and clinician engagement, we adopt a co-design approach where AI serves an assistive role, with final diagnoses or treatment plans emerging from collaboration between clinicians and AI.
Detection of Unknown-Unknowns in Cyber-Physical Systems using Statistical Conformance with Physics Guided Process Models
Sep 05, 2023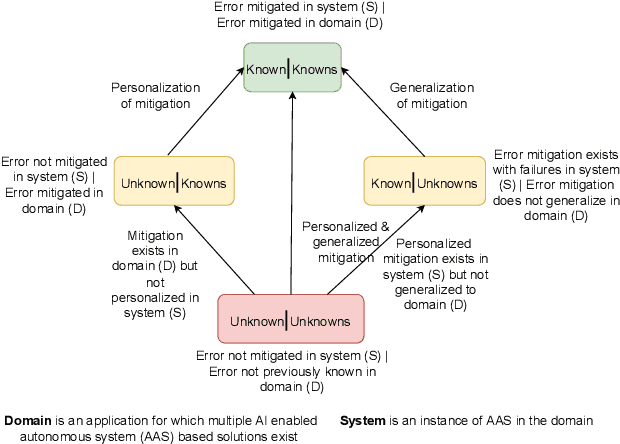
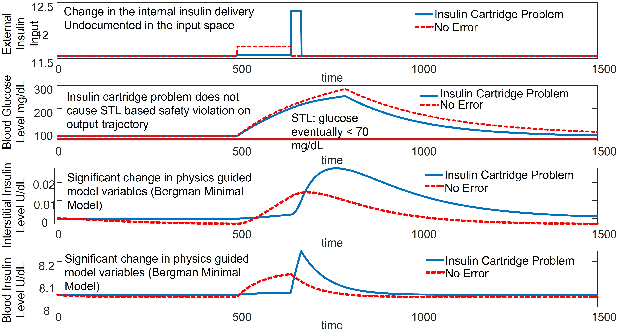
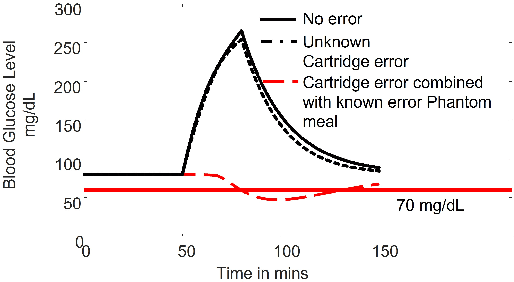
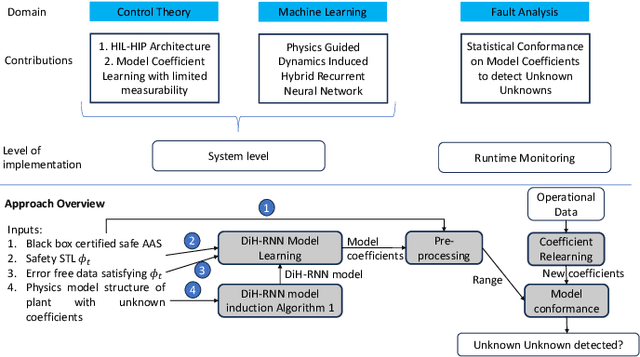
Abstract:Unknown unknowns are operational scenarios in a cyber-physical system that are not accounted for in the design and test phase. As such under unknown-unknown scenarios, the operational behavior of the CPS is not guaranteed to meet requirements such as safety and efficacy specified using Signal Temporal Logic (STL) on the output trajectories. We propose a novel framework for analyzing the stochastic conformance of operational output characteristics of safety-critical cyber-physical systems that can discover unknown-unknown scenarios and evaluate potential safety hazards. We propose dynamics-induced hybrid recurrent neural networks (DiH-RNN) to mine a physics-guided surrogate model (PGSM) which is used to check the model conformance using STL on the model coefficients. We demonstrate the detection of operational changes in an Artificial Pancreas(AP) due to unknown insulin cartridge errors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge