Pascal Haigron
Computer-aided shape features extraction and regression models for predicting the ascending aortic aneurysm growth rate
Mar 04, 2025



Abstract:Objective: ascending aortic aneurysm growth prediction is still challenging in clinics. In this study, we evaluate and compare the ability of local and global shape features to predict ascending aortic aneurysm growth. Material and methods: 70 patients with aneurysm, for which two 3D acquisitions were available, are included. Following segmentation, three local shape features are computed: (1) the ratio between maximum diameter and length of the ascending aorta centerline, (2) the ratio between the length of external and internal lines on the ascending aorta and (3) the tortuosity of the ascending tract. By exploiting longitudinal data, the aneurysm growth rate is derived. Using radial basis function mesh morphing, iso-topological surface meshes are created. Statistical shape analysis is performed through unsupervised principal component analysis (PCA) and supervised partial least squares (PLS). Two types of global shape features are identified: three PCA-derived and three PLS-based shape modes. Three regression models are set for growth prediction: two based on gaussian support vector machine using local and PCA-derived global shape features; the third is a PLS linear regression model based on the related global shape features. The prediction results are assessed and the aortic shapes most prone to growth are identified. Results: the prediction root mean square error from leave-one-out cross-validation is: 0.112 mm/month, 0.083 mm/month and 0.066 mm/month for local, PCA-based and PLS-derived shape features, respectively. Aneurysms close to the root with a large initial diameter report faster growth. Conclusion: global shape features might provide an important contribution for predicting the aneurysm growth.
Beyond Strong labels: Weakly-supervised Learning Based on Gaussian Pseudo Labels for The Segmentation of Ellipse-like Vascular Structures in Non-contrast CTs
Feb 05, 2024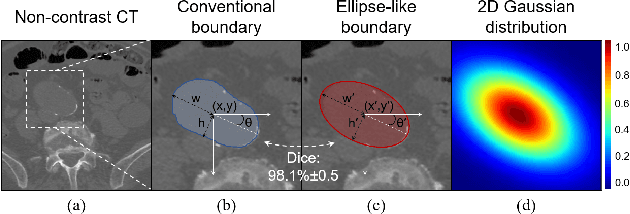

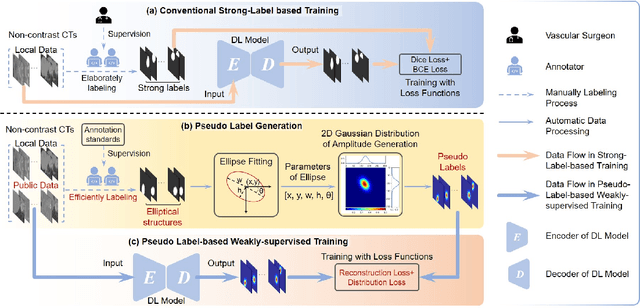
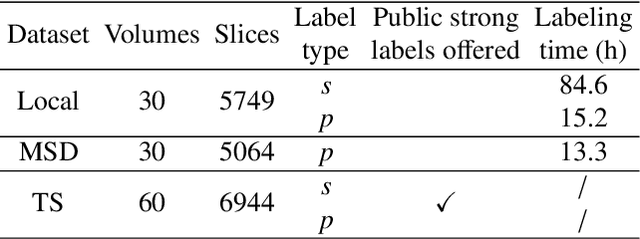
Abstract:Deep-learning-based automated segmentation of vascular structures in preoperative CT scans contributes to computer-assisted diagnosis and intervention procedure in vascular diseases. While CT angiography (CTA) is the common standard, non-contrast CT imaging is significant as a contrast-risk-free alternative, avoiding complications associated with contrast agents. However, the challenges of labor-intensive labeling and high labeling variability due to the ambiguity of vascular boundaries hinder conventional strong-label-based, fully-supervised learning in non-contrast CTs. This paper introduces a weakly-supervised framework using ellipses' topology in slices, including 1) an efficient annotation process based on predefined standards, 2) ellipse-fitting processing, 3) the generation of 2D Gaussian heatmaps serving as pseudo labels, 4) a training process through a combination of voxel reconstruction loss and distribution loss with the pseudo labels. We assess the effectiveness of the proposed method on one local and two public datasets comprising non-contrast CT scans, particularly focusing on the abdominal aorta. On the local dataset, our weakly-supervised learning approach based on pseudo labels outperforms strong-label-based fully-supervised learning (1.54\% of Dice score on average), reducing labeling time by around 82.0\%. The efficiency in generating pseudo labels allows the inclusion of label-agnostic external data in the training set, leading to an additional improvement in performance (2.74\% of Dice score on average) with a reduction of 66.3\% labeling time, where the labeling time remains considerably less than that of strong labels. On the public dataset, the pseudo labels achieve an overall improvement of 1.95\% in Dice score for 2D models while a reduction of 11.65 voxel spacing in Hausdorff distance for 3D model.
Deep Supervision by Gaussian Pseudo-label-based Morphological Attention for Abdominal Aorta Segmentation in Non-Contrast CTs
Feb 04, 2024Abstract:The segmentation of the abdominal aorta in non-contrast CT images is a non-trivial task for computer-assisted endovascular navigation, particularly in scenarios where contrast agents are unsuitable. While state-of-the-art deep learning segmentation models have been proposed recently for this task, they are trained on manually annotated strong labels. However, the inherent ambiguity in the boundary of the aorta in non-contrast CT may undermine the reliability of strong labels, leading to potential overfitting risks. This paper introduces a Gaussian-based pseudo label, integrated into conventional deep learning models through deep supervision, to achieve Morphological Attention (MA) enhancement. As the Gaussian pseudo label retains the morphological features of the aorta without explicitly representing its boundary distribution, we suggest that it preserves aortic morphology during training while mitigating the negative impact of ambiguous boundaries, reducing the risk of overfitting. It is introduced in various 2D/3D deep learning models and validated on our local data set of 30 non-contrast CT volumes comprising 5749 CT slices. The results underscore the effectiveness of MA in preserving the morphological characteristics of the aorta and addressing overfitting concerns, thereby enhancing the performance of the models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge