Orr Spiegel
An Empirically-parametrized Spatio-Temporal Extended-SIR Model for Combined Dilution and Vaccination Mitigation for Rabies Outbreaks in Wild Jackals
Jan 26, 2025Abstract:The transmission of zoonotic diseases between animals and humans poses an increasing threat. Rabies is a prominent example with various instances globally, facilitated by a surplus of meso-predators (commonly, facultative synanthropic species e.g., golden jackals [Canis aureus, hereafter jackals]) thanks to the abundance of anthropogenic resources leading to dense populations close to human establishments. To mitigate rabies outbreaks and prevent human infections, authorities target the jackal which is the main rabies vector in many regions, through the dissemination of oral vaccines in known jackals' activity centers, as well as opportunistic culling to reduce population density. Because dilution (i.e., culling) is not selective towards sick or un-vaccinated individuals, these two complementary epizootic intervention policies (EIPs) can interfere with each other. Nonetheless, there is only limited examination of the interactive effectiveness of these EIPs and their potential influence on rabies epizootic spread dynamics, highlighting the need to understand these measures and the spread of rabies in wild jackals. In this study, we introduce a novel spatio-temporal extended-SIR (susceptible-infected-recovered) model with a graph-based spatial framework for evaluating mitigation efficiency. We implement the model in a case study using a jackal population in northern Israel, and using spatial and movement data collected by Advanced Tracking and Localization of Animals in real-life Systems (ATLAS) telemetry. An agent-based simulation approach allows us to explore various biologically-realistic scenarios, and assess the impact of different EIPs configurations. Our model suggests that under biologically-realistic underlying assumptions and scenarios, the effectiveness of both EIPs is not influenced much by the jackal population size but is sensitive to their dispersal between activity centers.
Individual Variation Affects Outbreak Magnitude and Predictability in an Extended Multi-Pathogen SIR Model of Pigeons Vising Dairy Farms
Oct 12, 2023
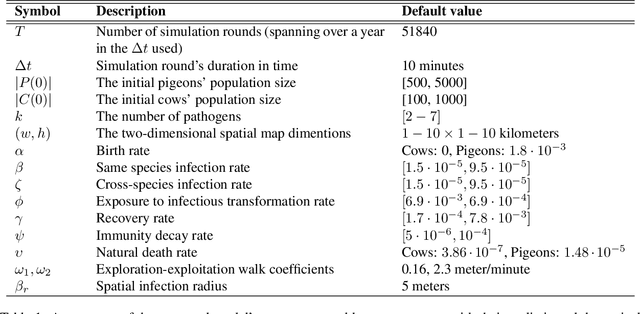
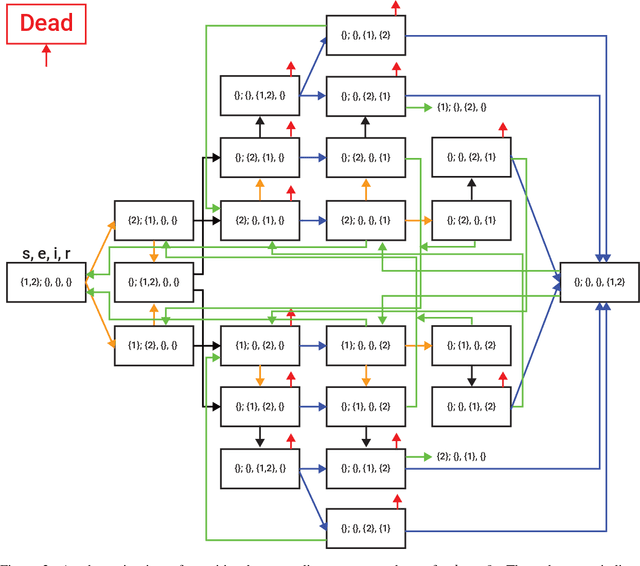
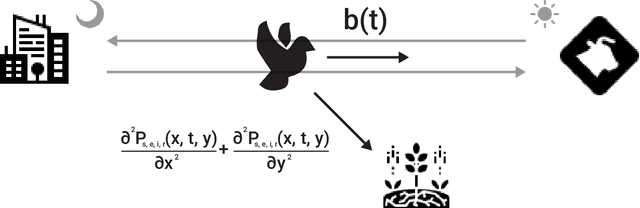
Abstract:Zoonotic disease transmission between animals and humans is a growing risk and the agricultural context acts as a likely point of transition, with individual heterogeneity acting as an important contributor. Thus, understanding the dynamics of disease spread in the wildlife-livestock interface is crucial for mitigating these risks of transmission. Specifically, the interactions between pigeons and in-door cows at dairy farms can lead to significant disease transmission and economic losses for farmers; putting livestock, adjacent human populations, and other wildlife species at risk. In this paper, we propose a novel spatio-temporal multi-pathogen model with continuous spatial movement. The model expands on the Susceptible-Exposed-Infected-Recovered-Dead (SEIRD) framework and accounts for both within-species and cross-species transmission of pathogens, as well as the exploration-exploitation movement dynamics of pigeons, which play a critical role in the spread of infection agents. In addition to model formulation, we also implement it as an agent-based simulation approach and use empirical field data to investigate different biologically realistic scenarios, evaluating the effect of various parameters on the epidemic spread. Namely, in agreement with theoretical expectations, the model predicts that the heterogeneity of the pigeons' movement dynamics can drastically affect both the magnitude and stability of outbreaks. In addition, joint infection by multiple pathogens can have an interactive effect unobservable in single-pathogen SIR models, reflecting a non-intuitive inhibition of the outbreak. Our findings highlight the impact of heterogeneity in host behavior on their pathogens and allow realistic predictions of outbreak dynamics in the multi-pathogen wildlife-livestock interface with consequences to zoonotic diseases in various systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge