Norbert Venantius Kang
Separation of Neural Drives to Muscles from Transferred Polyfunctional Nerves using Implanted Micro-electrode Arrays
Oct 14, 2024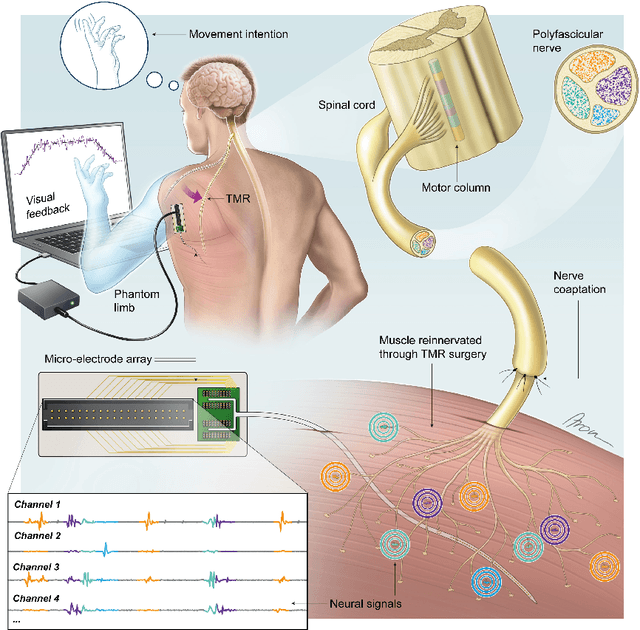
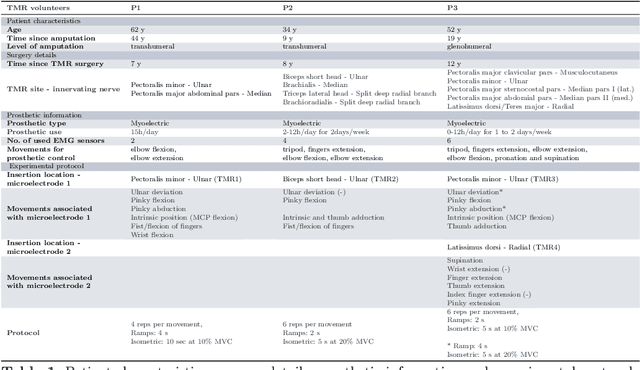
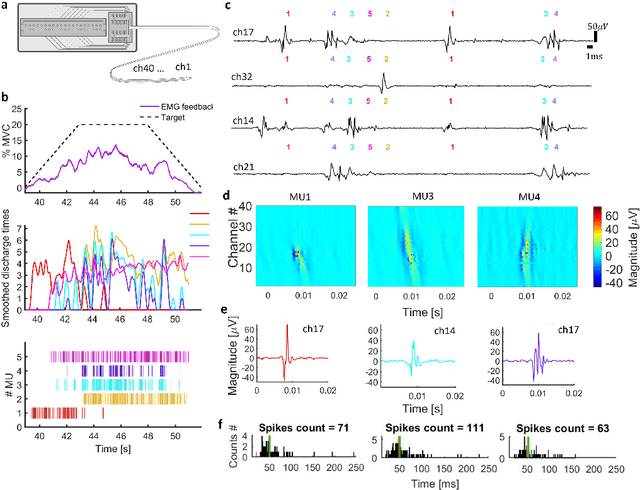

Abstract:Following limb amputation, neural signals for limb functions persist in the residual peripheral nerves. Targeted muscle reinnervation (TMR) allows to redirected these signals into spare muscles to recover the neural information through electromyography (EMG). However, a significant challenge arises in separating distinct neural commands redirected from the transferred nerves to the muscles. Disentangling overlapping signals from EMG recordings remains complex, as they can contain mixed neural information that complicates limb function interpretation. To address this challenge, Regenerative Peripheral Nerve Interfaces (RPNIs) surgically partition the nerve into individual fascicles that reinnervate specific muscle grafts, isolating distinct neural sources for more precise control and interpretation of EMG signals. We introduce a novel biointerface that combines TMR surgery of polyvalent nerves with a high-density micro-electrode array implanted at a single site within a reinnervated muscle. Instead of surgically identifying distinct nerve fascicles, our approach separates all neural signals that are re-directed into a single muscle, using the high spatio-temporal selectivity of the micro-electrode array and mathematical source separation methods. We recorded EMG signals from four reinnervated muscles while volunteers performed phantom limb tasks. The decomposition of these signals into motor unit activity revealed distinct clusters of motor neurons associated with diverse functional tasks. Notably, our method enabled the extraction of multiple neural commands within a single reinnervated muscle, eliminating the need for surgical nerve division. This approach not only has the potential of enhancing prosthesis control but also uncovers mechanisms of motor neuron synergies following TMR, providing valuable insights into how the central nervous system encodes movement after reinnervation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge