Nathalie Rochefort
Neuronal Learning Analysis using Cycle-Consistent Adversarial Networks
Nov 25, 2021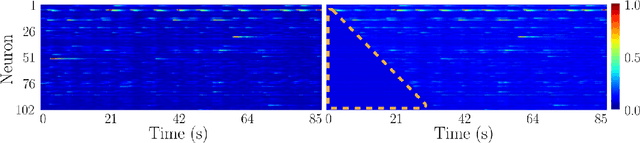
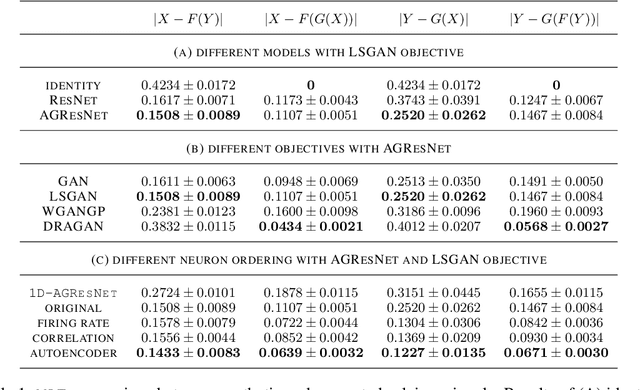
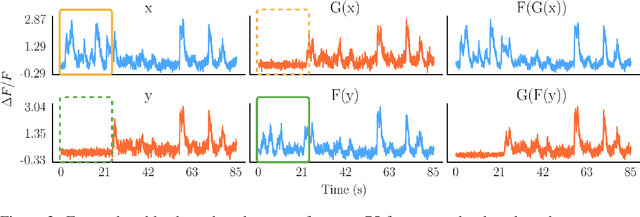
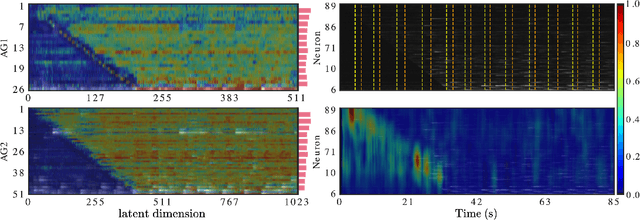
Abstract:Understanding how activity in neural circuits reshapes following task learning could reveal fundamental mechanisms of learning. Thanks to the recent advances in neural imaging technologies, high-quality recordings can be obtained from hundreds of neurons over multiple days or even weeks. However, the complexity and dimensionality of population responses pose significant challenges for analysis. Existing methods of studying neuronal adaptation and learning often impose strong assumptions on the data or model, resulting in biased descriptions that do not generalize. In this work, we use a variant of deep generative models called - CycleGAN, to learn the unknown mapping between pre- and post-learning neural activities recorded $\textit{in vivo}$. We develop an end-to-end pipeline to preprocess, train and evaluate calcium fluorescence signals, and a procedure to interpret the resulting deep learning models. To assess the validity of our method, we first test our framework on a synthetic dataset with known ground-truth transformation. Subsequently, we applied our method to neural activities recorded from the primary visual cortex of behaving mice, where the mice transition from novice to expert-level performance in a visual-based virtual reality experiment. We evaluate model performance on generated calcium signals and their inferred spike trains. To maximize performance, we derive a novel approach to pre-sort neurons such that convolutional-based networks can take advantage of the spatial information that exists in neural activities. In addition, we incorporate visual explanation methods to improve the interpretability of our work and gain insights into the learning process as manifested in the cellular activities. Together, our results demonstrate that analyzing neuronal learning processes with data-driven deep unsupervised methods holds the potential to unravel changes in an unbiased way.
CalciumGAN: A Generative Adversarial Network Model for Synthesising Realistic Calcium Imaging Data of Neuronal Populations
Sep 08, 2020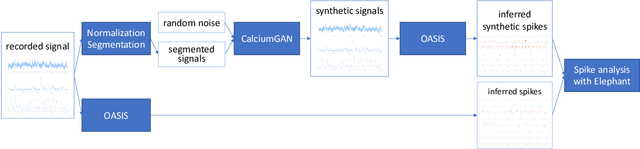
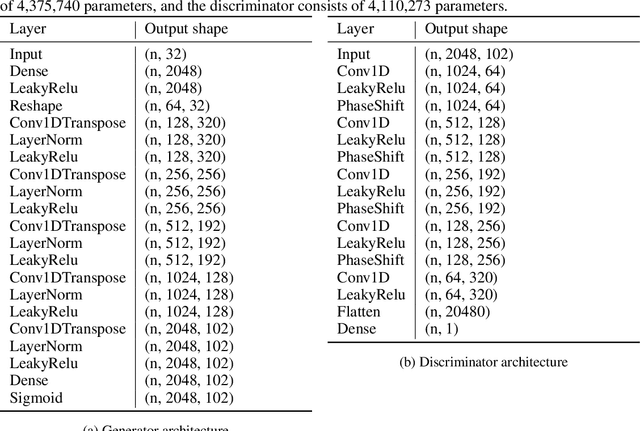
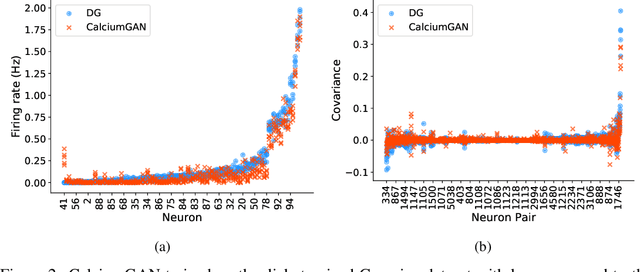
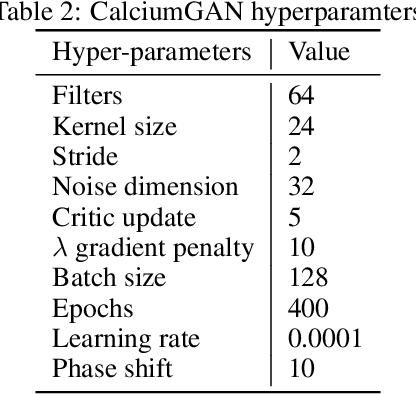
Abstract:Calcium imaging has become a powerful and popular technique to monitor the activity of large populations of neurons in vivo. However, for ethical considerations and despite recent technical developments, recordings are still constrained to a limited number of trials and animals. This limits the amount of data available from individual experiments and hinders the development of analysis techniques and models for more realistic size of neuronal populations. The ability to artificially synthesize realistic neuronal calcium signals could greatly alleviate this problem by scaling up the number of trials. Here we propose a Generative Adversarial Network (GAN) model to generate realistic calcium signals as seen in neuronal somata with calcium imaging. To this end, we adapt the WaveGAN architecture and train it with the Wasserstein distance. We test the model on artificial data with known ground-truth and show that the distribution of the generated signals closely resembles the underlying data distribution. Then, we train the model on real calcium signals recorded from the primary visual cortex of behaving mice and confirm that the deconvolved spike trains match the statistics of the recorded data. Together, these results demonstrate that our model can successfully generate realistic calcium imaging data, thereby providing the means to augment existing datasets of neuronal activity for enhanced data exploration and modeling.
Parametric Copula-GP model for analyzing multidimensional neuronal and behavioral relationships
Aug 03, 2020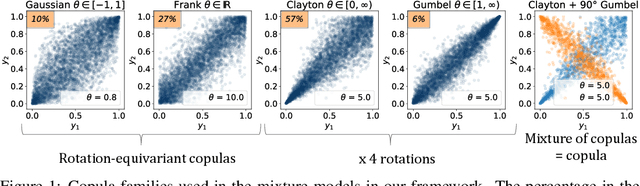
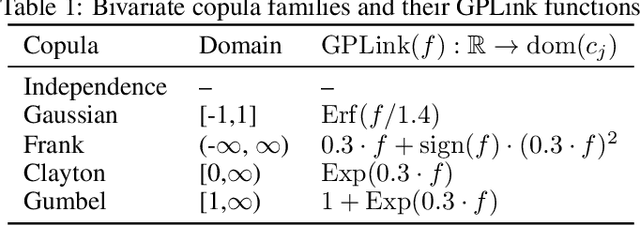
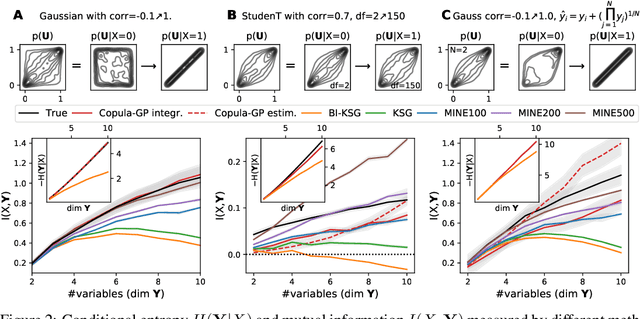
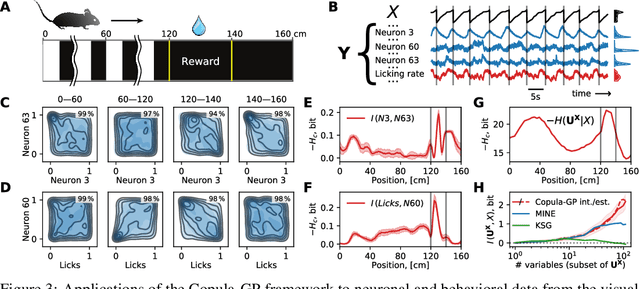
Abstract:One of the main challenges in current systems neuroscience is the analysis of high-dimensional neuronal and behavioral data that are characterized by different statistics and timescales of the recorded variables. We propose a parametric copula model which separates the statistics of the individual variables from their dependence structure, and escapes the curse of dimensionality by using vine copula constructions. We use a Bayesian framework with Gaussian Process (GP) priors over copula parameters, conditioned on a continuous task-related variable. We validate the model on synthetic data and compare its performance in estimating mutual information against the commonly used non-parametric algorithms. Our model provides accurate information estimates when the dependencies in the data match the parametric copulas used in our framework. When the exact density estimation with a parametric model is not possible, our Copula-GP model is still able to provide reasonable information estimates, close to the ground truth and comparable to those obtained with a neural network estimator. Finally, we apply our framework to real neuronal and behavioral recordings obtained in awake mice. We demonstrate the ability of our framework to 1) produce accurate and interpretable bivariate models for the analysis of inter-neuronal noise correlations or behavioral modulations; 2) expand to more than 100 dimensions and measure information content in the whole-population statistics. These results demonstrate that the Copula-GP framework is particularly useful for the analysis of complex multidimensional relationships between neuronal, sensory and behavioral data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge