Mohammad Mahmudul Hasan
A Novel Low-Complexity Peak-Power-Assisted Data-Aided Channel Estimation Scheme for MIMO-OFDM Wireless Systems
Oct 08, 2024Abstract:This paper, for the first time, presents a low-complexity peak-power-assisted data-aided channel estimation (DACE) scheme for both single-input single-output (SISO) and multiple-input multiple-output orthogonal frequency division multiplexing (MIMO-OFDM) wireless systems. In OFDM, high peak-power levels occur when the subcarriers align in phase and constructively interfere with each other. The research proposes a peak-power-assisted channel estimation scheme that accurately selects peak-power carriers at the transmitter of an OFDM system and uses them as reliable carriers for the DACE scheme. By incorporating these reliable carriers with known pilot symbols as additional pilot signals, channel estimation accuracy significantly improves in MIMO-OFDM systems. This eliminates the need to determine reliable data symbols at the receiver, thereby significantly reducing the computational complexity of the system. However, high peak-powers are considered a major drawback in OFDM. In this work, we incorporate a companding technique to mitigate this issue and provide sufficient margin for the DACE scheme. The performance of the proposed DACE scheme is evaluated using both least square (LS) and linear minimum mean square error (LMMSE) channel estimators. In this regard, the proposed technique not only improves channel estimation accuracy but also enhances the spectral efficiency of the wireless system. It outperforms traditional channel estimators in terms of system mean square error (MSE) and bit-error-rate (BER) performance. It also reduces the pilot overhead by 50$\%$ compared to traditional channel estimators and provides bandwidth optimization for MIMO-OFDM systems. This makes it a promising solution for improving the performance and efficiency of wireless communication systems.
Demystifying Deep Learning Models for Retinal OCT Disease Classification using Explainable AI
Nov 06, 2021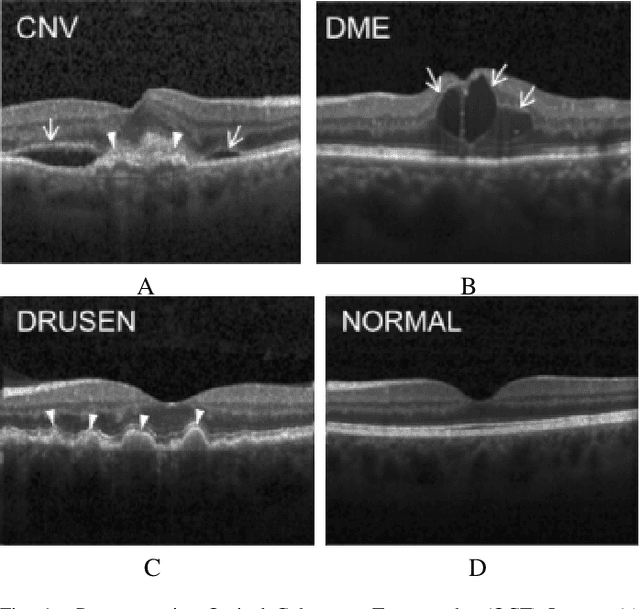
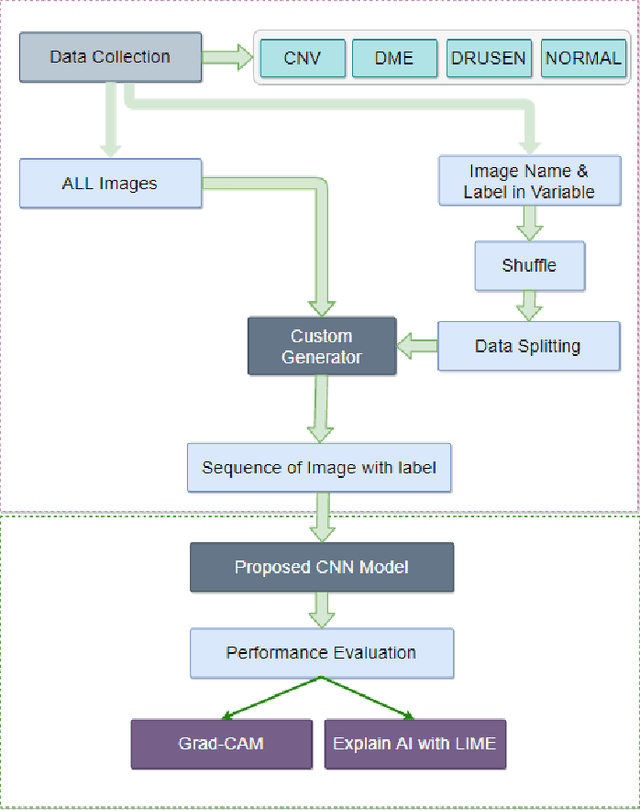
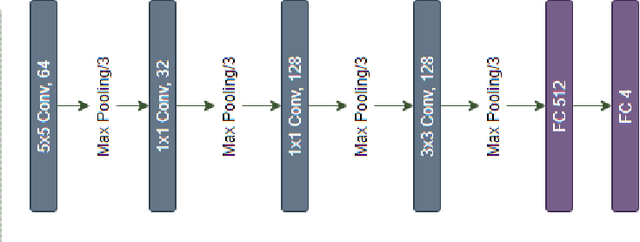
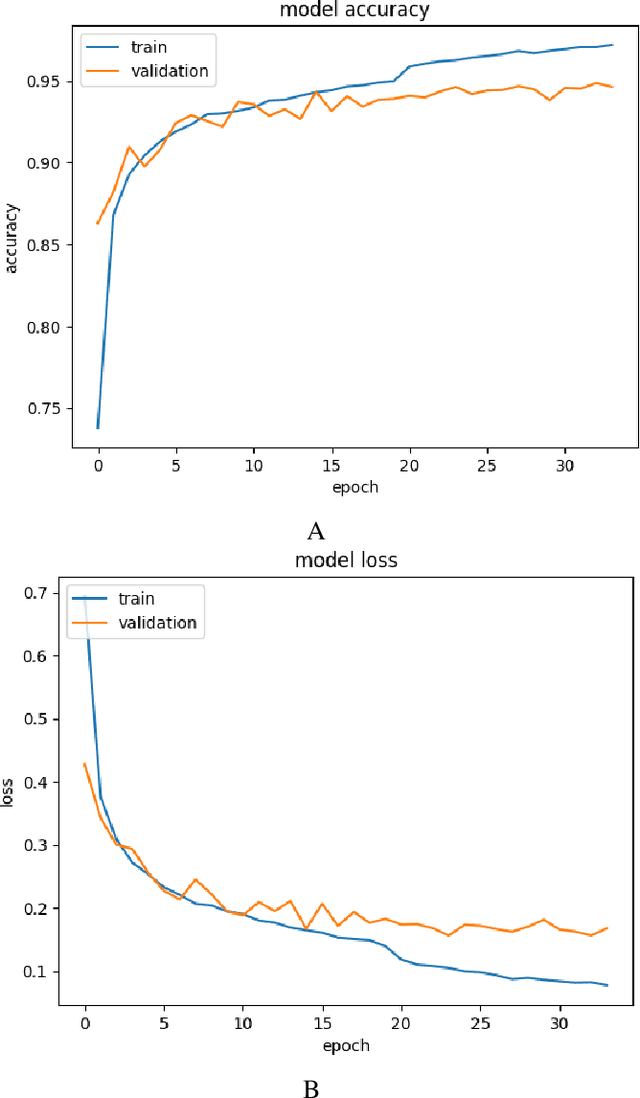
Abstract:In the world of medical diagnostics, the adoption of various deep learning techniques is quite common as well as effective, and its statement is equally true when it comes to implementing it into the retina Optical Coherence Tomography (OCT) sector, but (i)These techniques have the black box characteristics that prevent the medical professionals to completely trust the results generated from them (ii)Lack of precision of these methods restricts their implementation in clinical and complex cases (iii)The existing works and models on the OCT classification are substantially large and complicated and they require a considerable amount of memory and computational power, reducing the quality of classifiers in real-time applications. To meet these problems, in this paper a self-developed CNN model has been proposed which is comparatively smaller and simpler along with the use of Lime that introduces Explainable AI to the study and helps to increase the interpretability of the model. This addition will be an asset to the medical experts for getting major and detailed information and will help them in making final decisions and will also reduce the opacity and vulnerability of the conventional deep learning models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge