Martin J. O'Connor
Using association rule mining and ontologies to generate metadata recommendations from multiple biomedical databases
Mar 21, 2019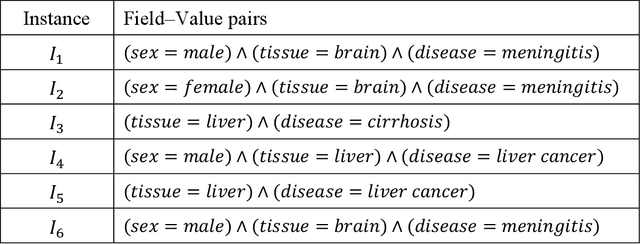

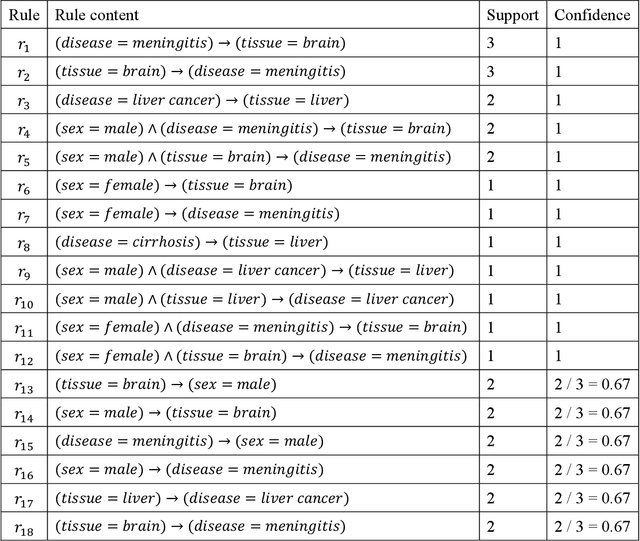
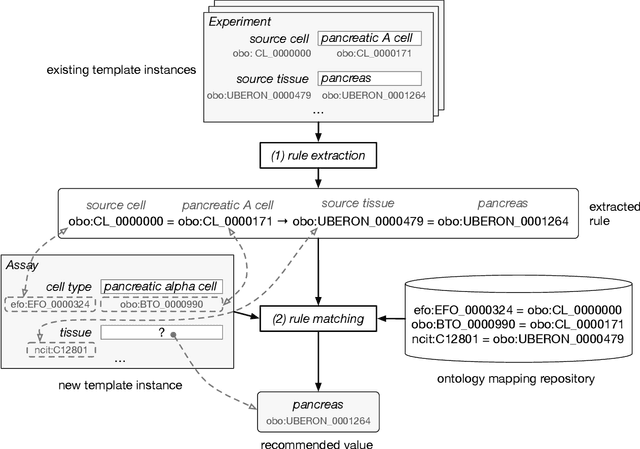
Abstract:Metadata-the machine-readable descriptions of the data-are increasingly seen as crucial for describing the vast array of biomedical datasets that are currently being deposited in public repositories. While most public repositories have firm requirements that metadata must accompany submitted datasets, the quality of those metadata is generally very poor. A key problem is that the typical metadata acquisition process is onerous and time consuming, with little interactive guidance or assistance provided to users. Secondary problems include the lack of validation and sparse use of standardized terms or ontologies when authoring metadata. There is a pressing need for improvements to the metadata acquisition process that will help users to enter metadata quickly and accurately. In this paper we outline a recommendation system for metadata that aims to address this challenge. Our approach uses association rule mining to uncover hidden associations among metadata values and to represent them in the form of association rules. These rules are then used to present users with real-time recommendations when authoring metadata. The novelties of our method are that it is able to combine analyses of metadata from multiple repositories when generating recommendations and can enhance those recommendations by aligning them with ontology terms. We implemented our approach as a service integrated into the CEDAR Workbench metadata authoring platform, and evaluated it using metadata from two public biomedical repositories: US-based National Center for Biotechnology Information (NCBI) BioSample and European Bioinformatics Institute (EBI) BioSamples. The results show that our approach is able to use analyses of previous entered metadata coupled with ontology-based mappings to present users with accurate recommendations when authoring metadata.
NCBO Ontology Recommender 2.0: An Enhanced Approach for Biomedical Ontology Recommendation
May 25, 2017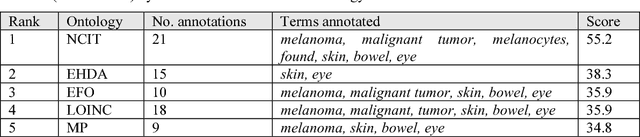
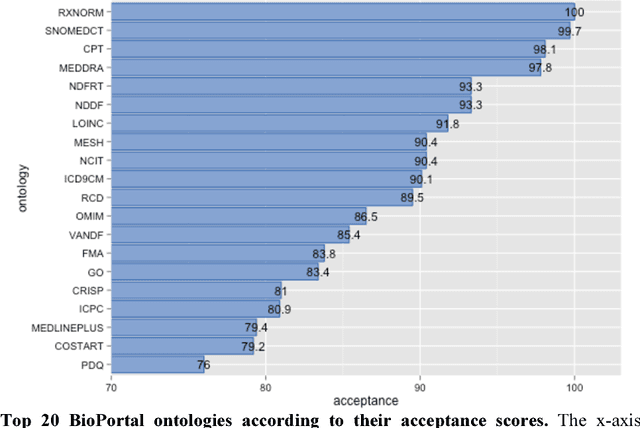
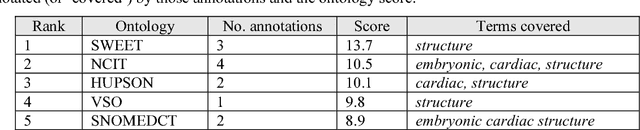
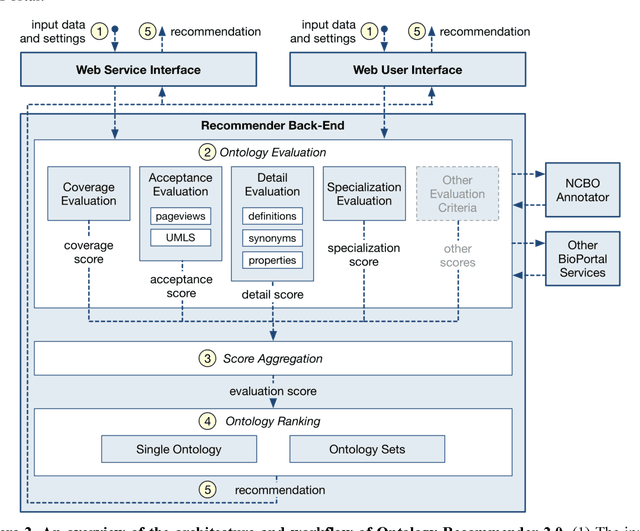
Abstract:Biomedical researchers use ontologies to annotate their data with ontology terms, enabling better data integration and interoperability. However, the number, variety and complexity of current biomedical ontologies make it cumbersome for researchers to determine which ones to reuse for their specific needs. To overcome this problem, in 2010 the National Center for Biomedical Ontology (NCBO) released the Ontology Recommender, which is a service that receives a biomedical text corpus or a list of keywords and suggests ontologies appropriate for referencing the indicated terms. We developed a new version of the NCBO Ontology Recommender. Called Ontology Recommender 2.0, it uses a new recommendation approach that evaluates the relevance of an ontology to biomedical text data according to four criteria: (1) the extent to which the ontology covers the input data; (2) the acceptance of the ontology in the biomedical community; (3) the level of detail of the ontology classes that cover the input data; and (4) the specialization of the ontology to the domain of the input data. Our evaluation shows that the enhanced recommender provides higher quality suggestions than the original approach, providing better coverage of the input data, more detailed information about their concepts, increased specialization for the domain of the input data, and greater acceptance and use in the community. In addition, it provides users with more explanatory information, along with suggestions of not only individual ontologies but also groups of ontologies. It also can be customized to fit the needs of different scenarios. Ontology Recommender 2.0 combines the strengths of its predecessor with a range of adjustments and new features that improve its reliability and usefulness. Ontology Recommender 2.0 recommends over 500 biomedical ontologies from the NCBO BioPortal platform, where it is openly available.
* 29 pages, 8 figures, 11 tables
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge