Joshua Hunte
A hybrid Bayesian network for medical device risk assessment and management
Sep 07, 2022



Abstract:ISO 14971 is the primary standard used for medical device risk management. While it specifies the requirements for medical device risk management, it does not specify a particular method for performing risk management. Hence, medical device manufacturers are free to develop or use any appropriate methods for managing the risk of medical devices. The most commonly used methods, such as Fault Tree Analysis (FTA), are unable to provide a reasonable basis for computing risk estimates when there are limited or no historical data available or where there is second-order uncertainty about the data. In this paper, we present a novel method for medical device risk management using hybrid Bayesian networks (BNs) that resolves the limitations of classical methods such as FTA and incorporates relevant factors affecting the risk of medical devices. The proposed BN method is generic but can be instantiated on a system-by-system basis, and we apply it to a Defibrillator device to demonstrate the process involved for medical device risk management during production and post-production. The example is validated against real-world data.
Product safety idioms: a method for building causal Bayesian networks for product safety and risk assessment
Jun 05, 2022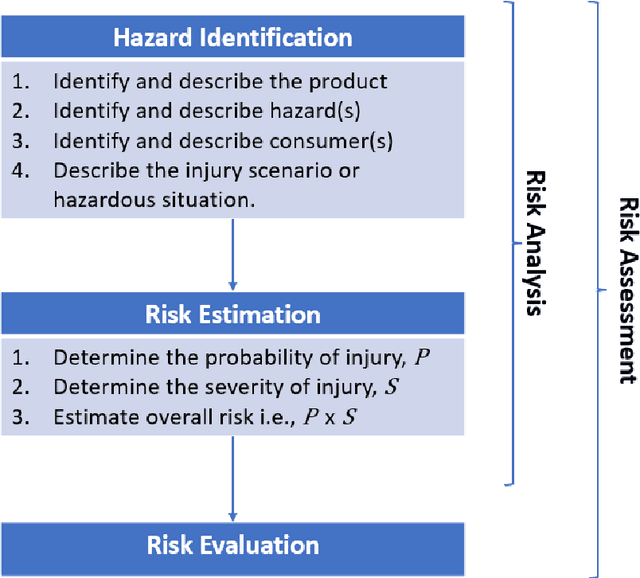
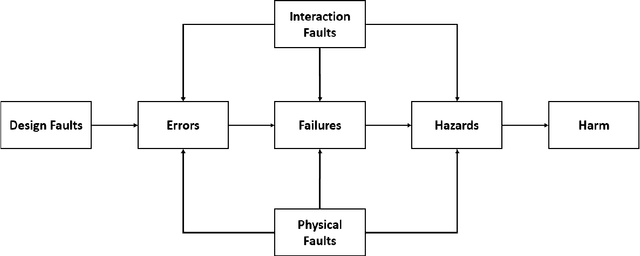
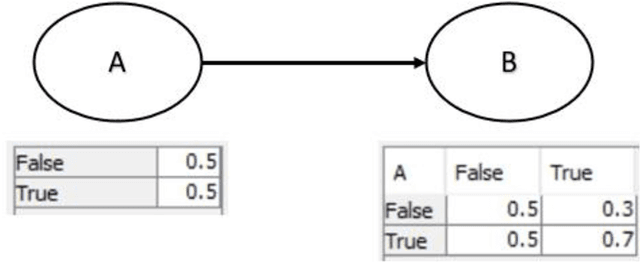
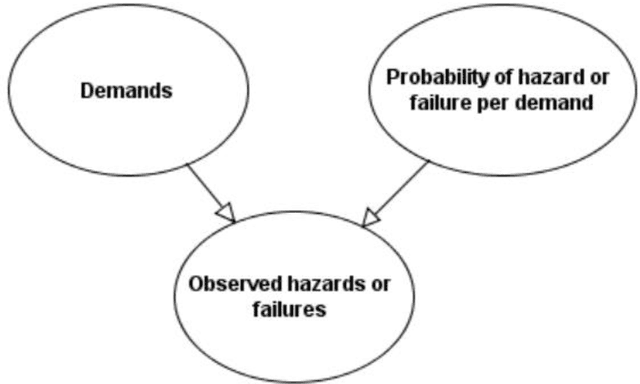
Abstract:Idioms are small, reusable Bayesian network (BN) fragments that represent generic types of uncertain reasoning. This paper shows how idioms can be used to build causal BNs for product safety and risk assessment that use a combination of data and knowledge. We show that the specific product safety idioms that we introduce are sufficient to build full BN models to evaluate safety and risk for a wide range of products. The resulting models can be used by safety regulators and product manufacturers even when there are limited (or no) product testing data.
Product risk assessment: a Bayesian network approach
Oct 09, 2020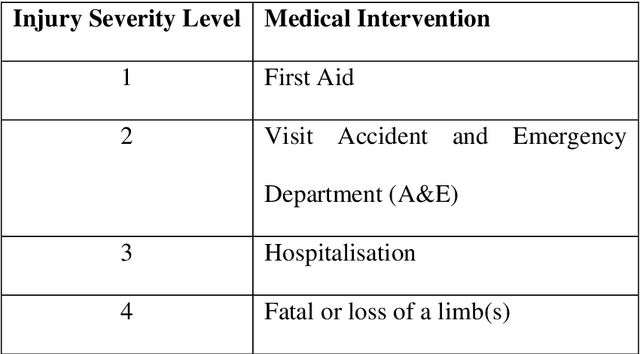
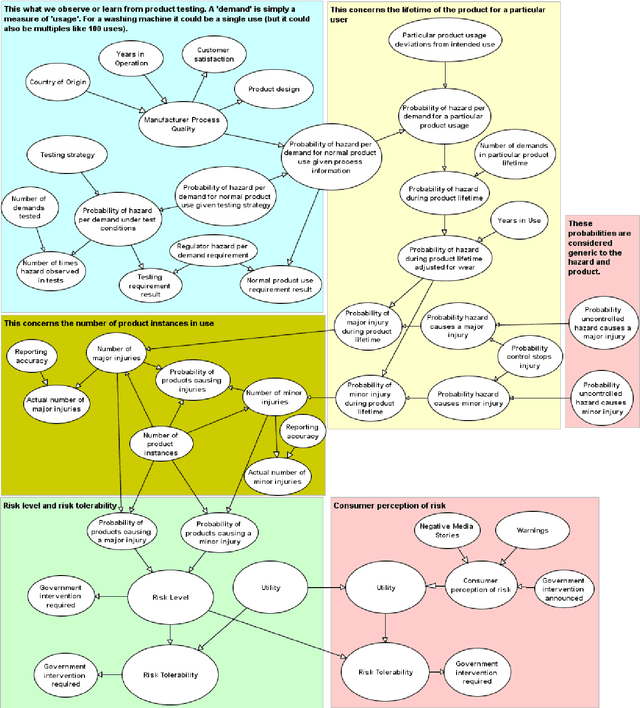
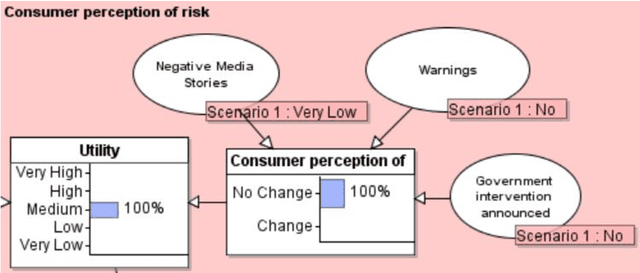
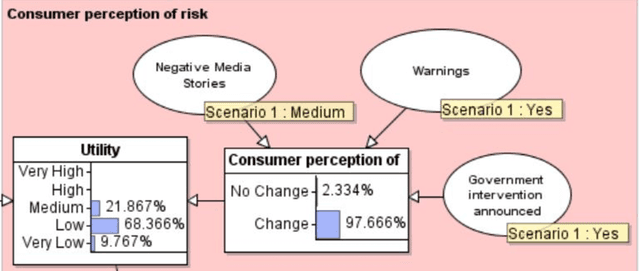
Abstract:Product risk assessment is the overall process of determining whether a product, which could be anything from a type of washing machine to a type of teddy bear, is judged safe for consumers to use. There are several methods used for product risk assessment, including RAPEX, which is the primary method used by regulators in the UK and EU. However, despite its widespread use, we identify several limitations of RAPEX including a limited approach to handling uncertainty and the inability to incorporate causal explanations for using and interpreting test data. In contrast, Bayesian Networks (BNs) are a rigorous, normative method for modelling uncertainty and causality which are already used for risk assessment in domains such as medicine and finance, as well as critical systems generally. This article proposes a BN model that provides an improved systematic method for product risk assessment that resolves the identified limitations with RAPEX. We use our proposed method to demonstrate risk assessments for a teddy bear and a new uncertified kettle for which there is no testing data and the number of product instances is unknown. We show that, while we can replicate the results of the RAPEX method, the BN approach is more powerful and flexible.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge