Ivan Olefir
Deep learning based electrical noise removal enables high spectral optoacoustic contrast in deep tissue
Feb 24, 2021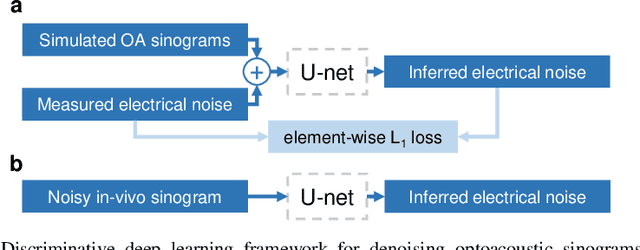
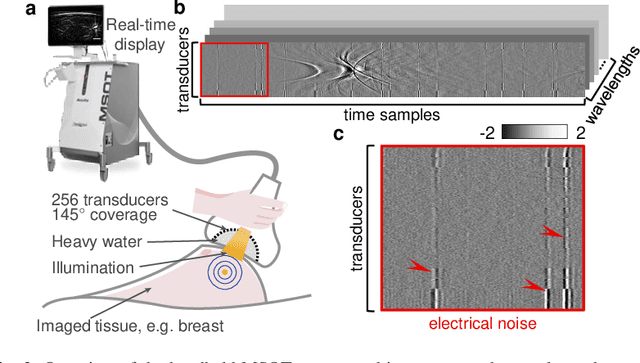
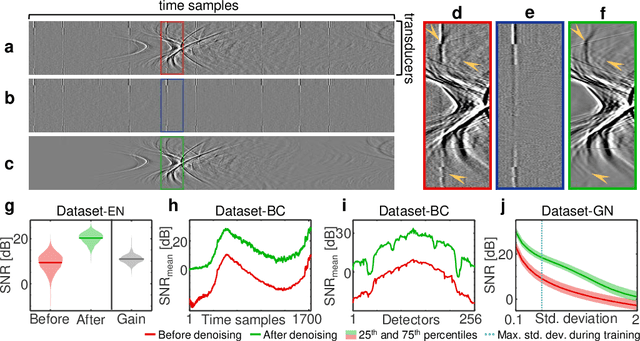
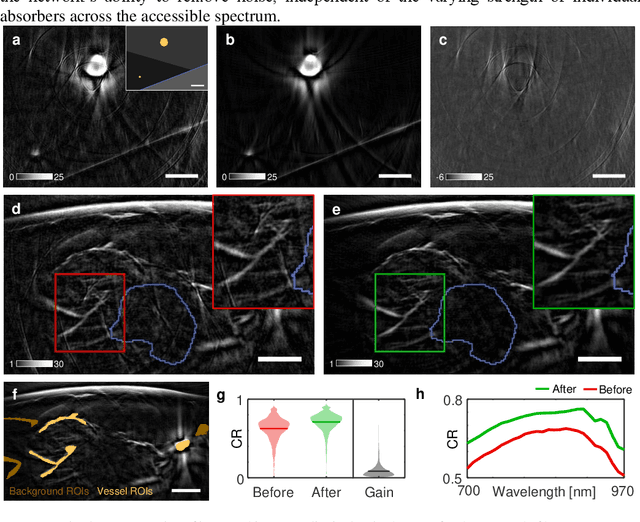
Abstract:Image contrast in multispectral optoacoustic tomography (MSOT) can be severely reduced by electrical noise and interference in the acquired optoacoustic signals. Signal processing techniques have proven insufficient to remove the effects of electrical noise because they typically rely on simplified models and fail to capture complex characteristics of signal and noise. Moreover, they often involve time-consuming processing steps that are unsuited for real-time imaging applications. In this work, we develop and demonstrate a discriminative deep learning (DL) approach to separate electrical noise from optoacoustic signals prior to image reconstruction. The proposed DL algorithm is based on two key features. First, it learns spatiotemporal correlations in both noise and signal by using the entire optoacoustic sinogram as input. Second, it employs training based on a large dataset of experimentally acquired pure noise and synthetic optoacoustic signals. We validated the ability of the trained model to accurately remove electrical noise on synthetic data and on optoacoustic images of a phantom and the human breast. We demonstrate significant enhancements of morphological and spectral optoacoustic images reaching 19% higher blood vessel contrast and localized spectral contrast at depths of more than 2 cm for images acquired in vivo. We discuss how the proposed denoising framework is applicable to clinical multispectral optoacoustic tomography and suitable for real-time operation.
Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues
Nov 18, 2015

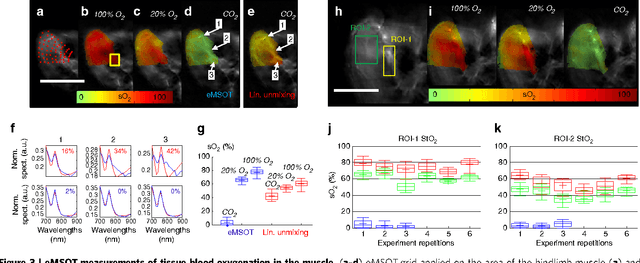

Abstract:Light propagating in tissue attains a spectrum that varies with location due to wavelength-dependent fluence attenuation by tissue optical properties, an effect that causes spectral corruption. Predictions of the spectral variations of light fluence in tissue are challenging since the spatial distribution of optical properties in tissue cannot be resolved in high resolution or with high accuracy by current methods. Spectral corruption has fundamentally limited the quantification accuracy of optical and optoacoustic methods and impeded the long sought-after goal of imaging blood oxygen saturation (sO2) deep in tissues; a critical but still unattainable target for the assessment of oxygenation in physiological processes and disease. We discover a new principle underlying light fluence in tissues, which describes the wavelength dependence of light fluence as an affine function of a few reference base spectra, independently of the specific distribution of tissue optical properties. This finding enables the introduction of a previously undocumented concept termed eigenspectra Multispectral Optoacoustic Tomography (eMSOT) that can effectively account for wavelength dependent light attenuation without explicit knowledge of the tissue optical properties. We validate eMSOT in more than 2000 simulations and with phantom and animal measurements. We find that eMSOT can quantitatively image tissue sO2 reaching in many occasions a better than 10-fold improved accuracy over conventional spectral optoacoustic methods. Then, we show that eMSOT can spatially resolve sO2 in muscle and tumor; revealing so far unattainable tissue physiology patterns. Last, we related eMSOT readings to cancer hypoxia and found congruence between eMSOT tumor sO2 images and tissue perfusion and hypoxia maps obtained by correlative histological analysis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge