Ilyes Benlala
Anatomical feature-prioritized loss for enhanced MR to CT translation
Oct 14, 2024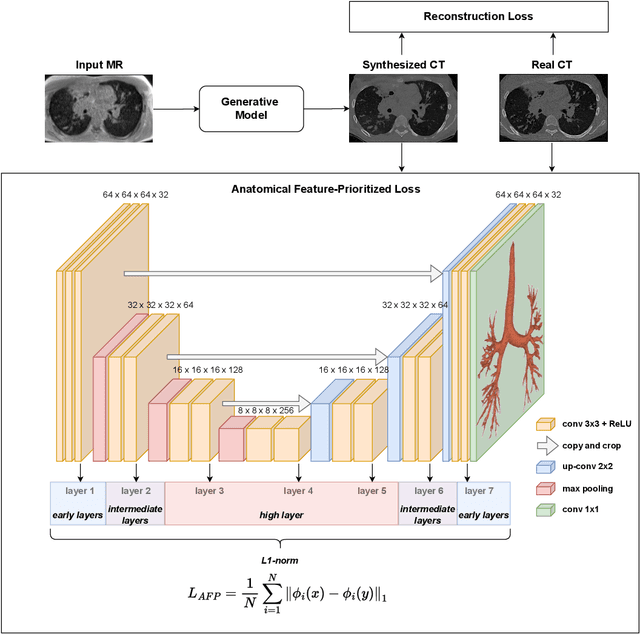
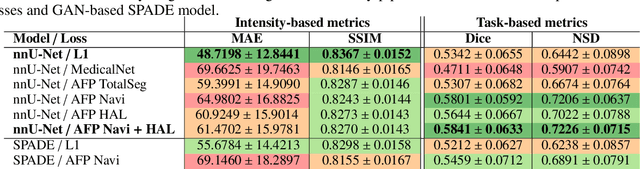
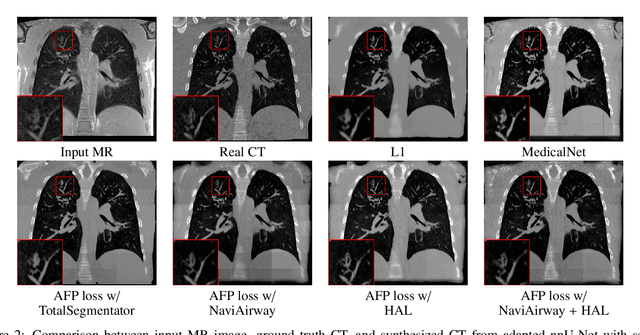
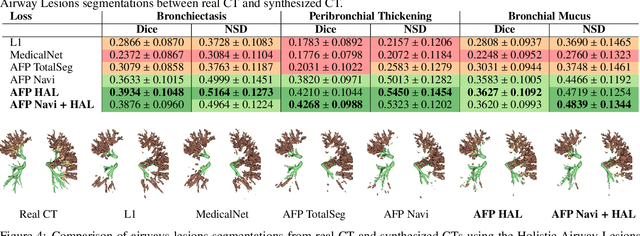
Abstract:In medical image synthesis, the precision of localized structural details is crucial, particularly when addressing specific clinical requirements such as the identification and measurement of fine structures. Traditional methods for image translation and synthesis are generally optimized for global image reconstruction but often fall short in providing the finesse required for detailed local analysis. This study represents a step toward addressing this challenge by introducing a novel anatomical feature-prioritized (AFP) loss function into the synthesis process. This method enhances reconstruction by focusing on clinically significant structures, utilizing features from a pre-trained model designed for a specific downstream task, such as the segmentation of particular anatomical regions. The AFP loss function can replace or complement global reconstruction methods, ensuring a balanced emphasis on both global image fidelity and local structural details. Various implementations of this loss function are explored, including its integration into different synthesis networks such as GAN-based and CNN-based models. Our approach is applied and evaluated in two contexts: lung MR to CT translation, focusing on high-quality reconstruction of bronchial structures, using a private dataset; and pelvis MR to CT synthesis, targeting the accurate representation of organs and muscles, utilizing a public dataset from the Synthrad2023 challenge. We leverage embeddings from pre-trained segmentation models specific to these anatomical regions to demonstrate the capability of the AFP loss to prioritize and accurately reconstruct essential features. This tailored approach shows promising potential for enhancing the specificity and practicality of medical image synthesis in clinical applications.
CT evaluation of 2D and 3D holistic deep learning methods for the volumetric segmentation of airway lesions
Mar 12, 2024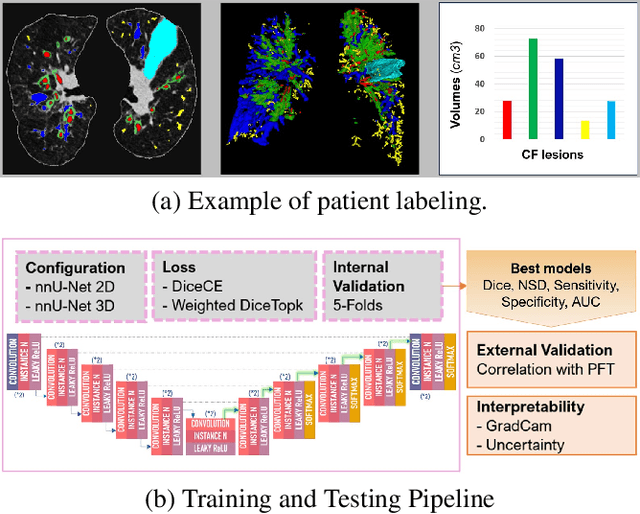
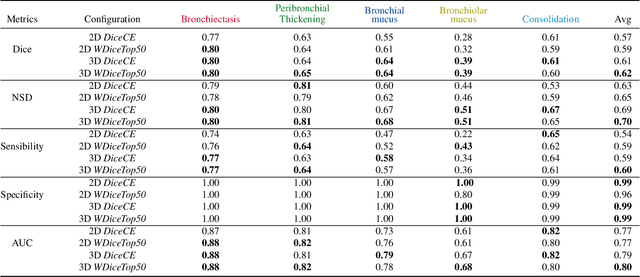
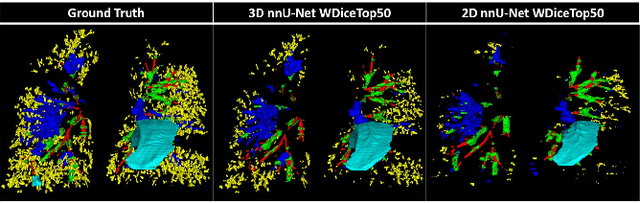
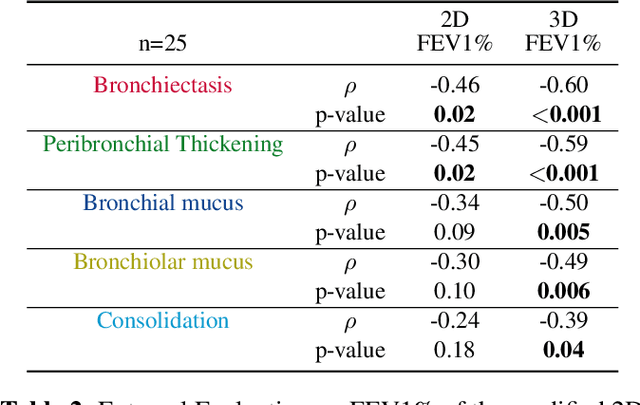
Abstract:This research embarked on a comparative exploration of the holistic segmentation capabilities of Convolutional Neural Networks (CNNs) in both 2D and 3D formats, focusing on cystic fibrosis (CF) lesions. The study utilized data from two CF reference centers, covering five major CF structural changes. Initially, it compared the 2D and 3D models, highlighting the 3D model's superior capability in capturing complex features like mucus plugs and consolidations. To improve the 2D model's performance, a loss adapted to fine structures segmentation was implemented and evaluated, significantly enhancing its accuracy, though not surpassing the 3D model's performance. The models underwent further validation through external evaluation against pulmonary function tests (PFTs), confirming the robustness of the findings. Moreover, this study went beyond comparing metrics; it also included comprehensive assessments of the models' interpretability and reliability, providing valuable insights for their clinical application.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge